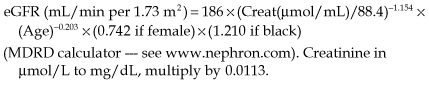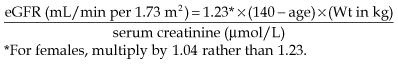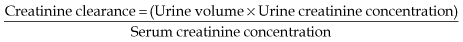10 Kidney and urinary tract disease
Approach to the Patient
Clinical presentations
Presentation with particular renal syndromes
Patients can present with the syndromes shown in Table 10.1.
Table 10.1 Presenting syndromes of renal disorders
| Syndrome | Clinical findings |
|---|---|
| Asymptomatic proteinuria ± microscopic haematuria | Protein and/or blood on urine dipstick ± renal impairment |
| Nephrotic syndrome | Proteinuria +++ Hypoalbuminaemia Peripheral oedema |
| Nephritic syndrome | Microscopic haematuria and proteinuria Renal impairment Hypertension |
| Rapidly progressive glomerulonephritis | Rapidly worsening renal function with red cell casts in an active urinary sediment |
| Macroscopic haematuria | Visible bloody discoloration of the urine |
Conditions that may be associated with renal disease
• Hypertension — may cause renal impairment and proteinuria, or be the consequence of renal disease.
• Drugs (NSAIDs, gold, penicillamine, antibiotics) — cause acute kidney injury, glomerulonephritis, tubulointerstitial nephritis.
Consequences of renal disease
• Uraemic symptoms. These do not develop until renal function is severely impaired (glomerular filtration rate (GFR) < 15 mL/min). Anorexia, nausea and vomiting, weight loss and loss of energy occur. Acidosis (with respiratory compensation) leads to shortness of breath. Confusion, fits and other neurological symptoms are signs of severe uraemia.
Examination of the urine
• Specific gravity (SG). SG is usually 1.003–1.035. As kidney disease progresses, the ability to concentrate the urine is lost. Urine may eventually have similar osmolality to plasma (which has SG = 1.010).
• Proteinuria. Normally the amount of protein in the urine should not exceed 150 mg/L, of which albumin should be < 20 mg/L. Significant proteinuria is a sign of glomerular disease, and usually consists predominantly of albumin (Bence Jones protein, as found in multiple myeloma, is an exception). ‘Nephrotic range proteinuria’ is a loose term, usually implying > 3 g/day of urine albumin loss and usually sufficient to cause hypoalbuminaemia. The urine dipstick is a sensitive marker of proteinuria. Quantification can be performed by measurement of:
• Microalbuminuria. Normal individuals excrete < 20 µg of albumin per min (30 mg in 24 hours) but dipsticks only detect levels above 200 µg (300 mg in 24 hours). The level between these two is called microalbuminuria. This is an early indicator of glomerular disease and a useful prognostic marker for future cardiovascular disease. Kits are available for testing.
• Albumin/creatinine ratio (ACR) can be tested on a random urine specimen. Because creatinine excretion is constant, correction by the creatinine concentration provides a correction for dilution. ACR may be useful in specific circumstances (e.g. early diabetic nephropathy). A ratio of 2.5–20 corresponds to albuminuria of 30–300 mg daily. Protein/creatinine ratio (PCR) is commonly measured if the dipstick is positive for protein.
• Haematuria. Red blood cells are not a normal finding in the urine. Between 5 and 10% of asymptomatic adults have microscopic haematuria. Causes include:
• confirm the presence of red cells in the urine (not routinely necessary if the dipstick is positive) and to exclude myoglobinuria (absence of red cells with dipstick-positive haematuria)
• diagnose red cell casts and dysmorphic red cells (normal morphology altered by passage through the renal tubules), pathognomonic of a glomerular cause of haematuria.
• Leucocyte esterase and nitrites. If white cells are present in the urine, then leucocyte esterase activity should be detectable. Nitrites are formed from nitrates by some bacteria. When both tests are positive, they are highly predictive of an acute urinary tract infection (UTI), with a sensitivity of 75% and specificity of 82%.
Evaluation of Renal Function
Glomerular disease
Measuring GFR
• Serum creatinine concentration. This provides a guide, but serum creatinine does not have a linear relationship with GFR. GFR will be as low as 50% of normal before the serum creatinine concentration rises above the upper limit of the ‘normal’ range.
• Equations to estimate GFR. Validated equations provide an estimated GFR (eGFR) and simplify the assessment of glomerular function. These are only appropriate for use in those with stable renal function (avoid in acute kidney injury). For the management of chronic kidney disease, eGFR is now the most useful measure of excretory renal function.
• Equations used to estimate GFR in clinical practice
• MDRD (Modification of Diet in Renal Disease) equation. This calculates GFR from 4–6 variables (including age, sex, race, serum creatinine concentration ± urea, albumin). It is not valid for use in patients with malnutrition or following limb amputation. Interpret it with caution at extremes of body weight. The four-variable MDRD equation is the most widely used eGFR measurement in clinical practice.
• Cockcroft–Gault equation. This is also well validated, but the formula requires an accurate weight.
Tubular disease
• phosphaturia, uricosuria and aminoaciduria (all markers of proximal tubular disease but rarely measured).
See p. 351 for the diagnosis and management of acute and chronic tubulointerstitial nephritis.
Imaging in renal disease
• Ultrasound (US) is non-invasive and relatively simple to perform. It provides information about the renal size (normally 9–13 cm; small kidneys are a sign of chronic kidney disease) and the cortical thickness (thin cortices are a sign of chronic renal damage; irregular cortices may suggest that scarring has occurred). Obstruction is shown by a dilated ureter and renal pelvis, and a bladder US can assess for incomplete voiding. A US can pick up anatomical abnormalities, e.g. cysts (single or multiple, benign or malignant), polycystic kidney disease, congenital absence of a kidney and other congenital abnormalities.
• Computed tomography of the kidneys, ureters and bladder (CT-KUB) is sensitive in the detection of renal stone disease. It is used for the differentiation of benign and potentially malignant cysts or renal masses. In obstruction, it may show the level of obstruction and the cause (extrinsic compression, stone disease, sloughed papilla, ureteric, bladder or prostatic tumour). It is used to stage renal and bladder tumours.
• Intravenous urography (IVU) provides anatomical detail of the calyces, renal pelvis, ureters and bladder. After a single injection of IV contrast, serial X-rays are taken of the kidneys, ureters and bladder. IVU is used (with a control plain film) in the diagnosis of stone disease, to diagnose or rule out tumours in the kidney or ureter, and to rule out anatomical abnormalities in recurrent UTI. It requires contrast and is contraindicated in moderate–severe renal impairment. Multiple films involve high exposure to ionizing radiation. IVU is time-consuming and relatively expensive, and has now been largely superseded by CT, which provides similar or more information in less time.
• Radio-isotope imaging uses the radio-isotopes DMSA (di-mercapto-succinic-acid), DTPA (diethylene-triamine-pentaacetic-acid) and MAG-3 (mercapto-acetyl-triglycine) to obtain functional and anatomical information. It:
• Diagnoses obstruction. DTPA or MAG-3 will be ‘held up’ on the obstructed side compared to the other side. This is useful if the US result is equivocal (a ‘baggy’ renal pelvis). Functional obstruction may be relieved after injection of diuretic, e.g. at the pelvi-ureteric junction.
• Magnetic resonance imaging (MRI) has a growing role in the assessment of renal tumours. It is useful in place of CT if contrast is contraindicated (renal impairment). MR angiography with gadolinium is a non-invasive, non-iodinated contrast used as an alternative to invasive angiography in the assessment of renovascular disease. Gadolinium should not be used in patients with renal insufficiency because of the risk of developing nephrogenic systemic fibrosis.
• Renal angiography is the gold standard investigation for renovascular disease. It can be combined with therapeutic angioplasty ± stent insertion. Complications include contrast-induced nephropathy (CO2 angiography is an alternative, though images are not as clear) and cholesterol emboli, and may worsen renal function.
Uroradiology
• Percutaneous nephrostomy involves percutaneous placement under US guidance of a nephrostomy tube; it can relieve obstruction. It can be followed by a nephrostogram (injection of contrast down the nephrostomy tube, followed by X-ray) to diagnose the site of obstruction.
• Urethroscopy, cystoscopy and ureteroscopy will allow diagnosis and surveillance of bladder tumours and the placement of retrograde stents in obstruction.
Renal biopsy
• Complications include bleeding (1% risk of requiring blood transfusion, 0.1% risk of death), clot colic with or without obstruction and pain (usually mild and transient).
• Indications include unexplained acute kidney injury, progressive chronic kidney disease where the cause is uncertain, nephrotic syndrome and nephritic syndrome.
• Relative contraindications include single (functioning) kidney, clotting or platelet abnormality (both should be checked pre-biopsy), small kidneys (likely to show end-stage glomerulosclerosis and tubulointerstitial fibrosis, and unlikely to result in any firm diagnosis or alter management) and uncontrolled hypertension, e.g. > 140/90 mmHg (increased risk of bleeding).
Urinary Tract Infection (UTI)
Glomerular Disease
Nephritic syndrome
• Presentation is with haematuria (usually microscopic), proteinuria, red cell casts, renal impairment (often progressive), hypertension and fluid retention (puffy face), with or without oliguria. These findings indicate inflammation of, and damage to, glomeruli and this is usually immune-mediated. If this is not reversed, irreversible glomerular loss and chronic kidney disease occur. An urgent renal biopsy will demonstrate glomerular nephropathy (GN) and, if rapidly progressive, may contain crescents (macrophage invasion of Bowman’s space, causing irreversible glomerular damage).
Nephrotic syndrome
Nephrotic syndrome presents with:
Nephrotic syndrome is the result of damage to the glomerular filtration barrier. The barrier is made up of the endothelium, the GBM, and podocytes and their slit diaphragms. Both molecular size and charge affect the selectivity of the barrier, which is disrupted in the nephrotic syndrome.
Investigations of glomerular disease
• Urine dipstick: microscopy if haematuria demonstrated (?red cell casts). Quantify proteinuria (p. 328).
• Serum electrolytes, urea, creatinine (serial measurements — progressive disease requires urgent investigation), bone profile, liver biochemistry, protein electrophoretic strip, blood glucose and lipid profile. Urine for Bence Jones protein.
• Nephritic screen: ANA, dsDNA, C3, C4, ANCA, anti-GBM, hepatitis B and C serology, blood cultures if fever or suggestion of sepsis.
Management of glomerular disease: general principles
• Salt and water balance. Accurate assessment of fluid balance is necessary (p. 368), as in severe nephrotic syndrome there may be intravascular depletion despite expansion of the extracellular compartment. Hypovolaemia should be corrected with oral or IV fluids. Volume expansion should be managed with salt and water restriction, with or without diuretics. High doses of loop diuretics are often required to establish and maintain a diuresis, e.g. furosemide 40–250 mg twice daily.
• BP. Hypertension is usual in glomerular disease. Reducing BP leads to decreased intraglomerular pressure, and decreased levels of proteinuria, preservation of renal function and protection of other organs from hypertension-induced damage. ACE inhibitors and/or angiotensin receptor blockers (ARBs) have theoretical and evidence-based advantages over other agents, especially for those with significant proteinuria. Target BP should be 130/80 mmHg (120/75 mmHg if there is proteinuria).
• Lipids. Statins are given to lower the serum cholesterol (< 4.5 mmol/L) (to convert mmol to mg/dL multiply by 38).
• Dialysis. This is required if renal impairment is severe. For indications for dialysis see p. 357. With definitive treatment and renal recovery, dialysis is discontinued after a period of time.
• Anticoagulants. Risk of venous thromboembolism is high; give prophylactic anticoagulants (p. 247).
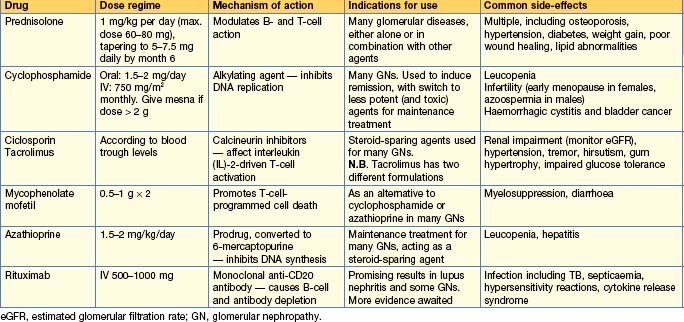
General principles for immunosuppressive treatment
• The decision to treat is based on balancing the potential benefits of the treatment with the potential risks. With potent immunosuppression, the side-effects may be life-threatening.
• Many treatment regimes use the principle of induction (with a potent drug combination) to induce ‘remission’ and then maintenance therapy with less toxic treatment to prevent relapse.
• Monitoring must be appropriate and robust (e.g. weekly or fortnightly blood tests initially when on cyclophosphamide or azathioprine, checks of GFR and monitoring of serum levels when on ciclosporin or tacrolimus).
• Use adjunctive therapies to minimize the risk of side-effects whenever possible. These include:
• Bone protection treatment for all on long-term steroids (yearly dual energy X-ray absorptiometry (DEXA) scans). Use a long-acting oral bisphosphonate (e.g. alendronate 5 mg daily) and/or calcium and vitamin D combinations (e.g. calcium carbonate 1.25 g and cholecalciferol 5 mcg).
• Co-trimoxazole 480 mg twice daily as prophylaxis against Pneumocystis jiroveci infection for patients on cyclophosphamide.
• Isoniazid 100 mg daily as anti-TB prophylaxis for at-risk patients (history of TB or TB exposure) on cyclophosphamide.
• All immunosuppressive regimes have an associated risk of infections and, in the longer term, malignancy. Other common side-effects are shown in Table 10.2.
Specific glomerular diseases and their management
Minimal change disease
• Renal biopsy shows normal appearances on light microscopy with no immune complex deposition (‘minimal change’). EM shows epithelial cell (podocyte) foot process fusion — a non-specific finding seen in any condition associated with the nephrotic syndrome.
• An immunological cause is suggested by the fact that the condition responds to immunosuppression. Production of a circulating factor (not yet identified) by T-cells or immature CD34-positive stem cells has been suggested.
• Spontaneous remissions occur, so treatment should not be commenced unless hypoalbuminaemia and oedema are present.
• Management
• Start with high-dose corticosteroids, i.e. prednisolone 1 mg/kg/day in adults or 60 mg/m2 in children up to a maximum of 80 mg/day for 4–6 weeks, and then 40 mg/m2 for 4–6 weeks. Remission may occur within days, but can take weeks.
• Once in remission, prednisolone dose should be reduced slowly (cut dose by 30% after 4–6 weeks). Steroids should be tapered and the patient weaned off them over a total of 12 more weeks.
• Relapse frequently occurs (but one-third of children will not relapse), especially if steroids are tapered too quickly, and this should be treated in the same way with prednisolone.
• Frequent relapsers may be steroid-dependent (relapse when steroids are withdrawn) or steroid-resistant (fail to go into remission with steroids). Cyclophosphamide (1.5–2 mg/kg/day) for 8–12 weeks given with prednisolone 7.5–15 mg/day increases the likelihood of long-term remission. Ciclosporin (3–5 mg/kg/day aiming for trough blood level of 80–150 ng/mL) is an alternative but needs to be continued long-term to prevent relapse; it carries its own risks of nephrotoxicity so frequent monitoring of levels and kidney function is required. Levamisole 2.5 mg/kg (max. 150 mg) on alternate days has been found to be effective in maintaining remission in children.
Focal segmental glomerulosclerosis (FSGS)
• Primary FSGS is of unknown cause. A circulating permeability factor with serine protease activity has been implicated (the disease may recur after transplantation, often immediately). A renal biopsy is required to make the diagnosis, determine therapy and indicate prognosis. Segmental scleroses in affected glomeruli are seen, with other glomeruli looking normal on light microscopy. C3 and IgM may be present on immunofluorescence in affected segments. Mesangial hypercellularity may be present. Interstitial fibrosis and focal tubal atrophy are common. On EM, affected glomeruli show capillary obliteration with hyaline deposits and lipids. Patchy foot process effacement is present, even on ‘normal’-looking glomeruli. Five histological types are described by light microscopy:
• Tip lesion — scleroses occur at the tubular pole of affected glomeruli, with foam cell-filled capillaries and adhesion of epithelial cells to the proximal portion of the tubule.
• Collapsing FSGS — with enlarged and vacuolated visceral cells, and collapsed capillary walls. This variant is often associated with HIV infection (especially in black people), when it is classified as HIV-associated nephropathy (HIVAN).
• Perihilar variant — with perihilar sclerosis and hyalinosis, frequently present in secondary FSGS (see below).
• Secondary FSGS occurs as a result of a loss of functioning nephrons of almost any cause, e.g. hypertension, obesity, previous nephrectomy or renal damage, with the remnant nephrons having to hyperfilter. This leads to hydraulic injury over time.
• Management. Mild to modest proteinuria should be managed with good BP control (ACE inhibitors), a statin and follow-up (Box 10.1). Overt nephrotic syndrome and/or progressive renal impairment (drop in eGFR of > 15% in 1 year or > 10% in 2 successive years) are risk factors for progression and indications for immunosuppression. Prednisolone 0.5–2 mg/kg/day should be continued for up to 6 months before steroid resistance is diagnosed. Commonly this is the case, and other disease-modifying drugs are required. Ciclosporin aiming for trough level 150–300 ng/mL may be effective, but relapse may occur when it is discontinued. Cyclophosphamide 1–1.5 mg/kg/day with high-dose prednisolone for 3–6 months, followed by maintenance treatment with prednisolone and azathioprine, may reduce proteinuria and slow progression, especially if there is mesangial hypercellularity and tip lesions. Chlorambucil has also been used with some success. Despite treatment, 50% progress to end-stage kidney disease within 10 years of diagnosis. HIVAN is managed with anti-retroviral therapy. Renal function may stabilize or improve.
Membranous glomerulonephritis
• In 75% of cases no underlying cause is found. Causes include drugs (e.g. gold, penicillamine, NSAIDs), autoimmune disease (e.g. SLE, thyroiditis), infections (e.g. hepatitis B or C, schistosomiasis, Plasmodium malariae, leprosy) and neoplasia (e.g. solid organ tumours and lymphomas).
• Pathology shows thickening of the capillary basement membrane on light microscopy and deposition of C3 and IgG on immunofluorescence. EM changes are detectable earlier in the course of the disease and mirror light microscopy changes, with electron-dense sub-epithelial capillary wall deposits. It is thought that immune complexes are formed in situ in the podocyte basement membrane and the target auto-antigen(s) in humans is the M-type phospholipase A2 receptor (PLA2R).
• Management (Box 10.1)
• If an underlying condition is present, it should be treated. This may slow or halt the progression of disease.
• In primary disease, spontaneous remission is common (up to 40%), so specific treatment should be withheld for up to 6 months unless there is progression. Even then, only those with severe proteinuria and/or progressive renal impairment should be treated with immunosuppression.
• For such high-risk patients, steroids are ineffective if given alone. Combinations which have been used with some success include:
a) Prednisolone 1 mg/kg/day on alternate days with cyclophosphamide 1.5–2.5 mg/kg/day for 6–12 months.
b) Chlorambucil 0.2 mg/kg/day in months 2, 4 and 6 with prednisolone 0.4 mg/kg/day in months 1, 3 and 5.
d) Rituximab, an anti-CD25 antibody that ablates B lymphocytes; this has been shown to improve renal function and reduce proteinuria without significant side-effects in short-term studies. Long-term outcome is not yet known.
The effectiveness of the various drug regimens is unclear, and good controlled trials are lacking.
Amyloidosis
• There are two types:




• AA amyloid is associated with chronic infections or inflammatory conditions (e.g. TB, bronchiectasis, IV drug use, rheumatoid arthritis, ankylosing spondylitis, familial Mediterranean fever, inflammatory bowel disease).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree