Qualitatively different from ACTH-(1–24)-elicited body groominga
Mechanistically different from the scratching associated with ODT8-SS, a somatostatin analogueb
Also observed after intraperiaqueductalb gray infusion and intrathecal administrationc but not after intravenous injection
Unaffected by either hypophysectomy or adrenalectomyb
Not markedly affected by behaviorally nondepressant doses of haloperidol, morphine, naloxone, haloperidol, or antihistaminesb
Provides a possible animal model for preclinical screening of potential antipruritic agentsd
Antagonized in a dose-dependent manner by agonists at kappa opioid receptorse
In the 1990s, more selective kappa agonists were being evaluated as analgesics, and the question arose at the bench level as to whether such second-generation compounds might also possess antiscratch activity. Enadoline is just one example from the arylacetamide class that was tested in phase II clinical trials but, like so many similar analgesics possessing that chemical scaffold, was never commercialized (Hunter et al. 1990; Reece et al. 1994; Pande et al. 1996). The main reason for such failures is well known to analgesic researchers and can be stated bluntly in the presence of two worrisome side effects that have been intimately linked with the evolution of kappa agonists over the years: dysphoria and psychotomimesis (Kumor et al. 1986; Pfeiffer et al. 1986; Walsh et al. 2001). Despite kappa agonists being perceived as offering greater safety than traditional morphine-like compounds, a view based on the milder form of physical dependence that develops in animals, and limited actions on respiration and gastrointestinal transit, the associated psychotoxicity has dimmed the clinical prospects of these agents as analgesics (and possibly as potential antipruritics). One response to this situation has been to investigate the pharmacological profiles of peripherally directed kappa agonists given that kappa opioid receptors are present on the peripheral terminals of primary afferent neurons. ICI 204,448 (Shaw et al. 1989), GR 94839 (Rogers et al. 1992), and asimadoline (Barber et al. 1994) are examples of kappa analgesics that are believed to pass across the blood barrier with great difficulty, if at all. We compared ICI 204,448, a diarylacetamide derivative, with the centrally penetrating enadoline in the following two experimental models: (1) the rat paw formalin test for persistent pain (Wheeler-Aceto and Cowan 1991) and (2) the rat bombesin test for antiscratch activity. The subcutaneous antinociceptive-50 (A50) value of enadoline against formalin-elicited late phase flinching was 0.19 mg/kg. The corresponding value for ICI 204,448 was 6.9 mg/kg which reflects suppression of inflammatory pain by means of peripheral kappa opioid receptors. The subcutaneous A50 value of enadoline against scratching elicited by bombesin was equally impressive (0.012 mg/kg), whereas ICI 204,448 showed no antipruritic potential in this procedure, the A50 value being greater than 30 mg/kg.
Results from this particular study reinforced the prevailing view that antagonism of bombesin-evoked scratching requires penetration across the blood–brain barrier and subsequent agonism at central kappa receptors, physicochemical attributes associated with enadoline but not with ICI 204,448. Inactivity against bombesin-induced scratching was nevertheless helpful for screening and characterizing in vivo those kappa agonists with the preferred peripheral locus of action. There was an obvious need for additional animal models that generate itch stimuli at local, peripheral sites. One such model made use of the pruritogenic properties of compound 48/80 in mice.
3 Compound 48/80-Induced Scratching in Mice
Pharmacologists and chemists were alerted to the possibility of establishing structure–activity relationships for potential antipruritic agents in mice with the publication of a standardized, apparently straightforward, and efficient methodology by Kuraishi and colleagues in 1995. Their approach did not involve the laborious procedure of cannulating the cerebroventricles of rats but, rather, depended on a single subcutaneous injection of pruritogen behind the neck of awake, ddY mice and the immediate monitoring of spontaneous bouts of scratching by hind paws at the injection site. Importantly, Kuraishi et al. (1995) concluded that the excessive scratching was associated with the sensation of itch (elicited by compound 48/80, a mast cell degranulator and pruritogen in humans) and not with the sensation of pain (provoked by either capsaicin or dilute formalin). The mechanism responsible for this repetitive behavior requires further study particularly since compound 48/80 still causes scratching in mast cell-deficient mice (Dunford et al. 2007) and, additionally, is only weakly active in Sprague Dawley rats (Thomsen et al. 2001).
The compound 48/80 behavioral test has face validity (symptom homology) and enjoyed subsequent popularity as an initial, general screen for detecting compounds with antiscratch activity in much the same way as the mouse abdominal constriction test (e.g., Pearl et al. 1968) has been used historically to obtain dose–response curves for possible antinociceptive agents. In both cases, activity in the screen may be taken as a positive indication of chemical progress, but implicating human itch or pain states would be premature in the absence of more comprehensive testing. That being said, it should be noted that Green et al. (2006) injected eleven inbred strains of mice with a different pruritogen (chloroquine, s.c., in the nape of the neck) and concluded that their results supported the utility of the Kuraishi model in the general area of itch research.
Our initial work was greatly influenced by findings from the Kuraishi group and involved assessing the antiscratch activities of subcutaneously administered opioids in Swiss Webster mice that were subsequently challenged with compound 48/80. Results from these early experiments revealed that neither naloxone (0.10–3 mg/kg) nor the standard kappa receptor antagonist, norbinaltorphimine (norBNI, 20 mg/kg at −15 h), had marked effects on compound 48/80-induced scratching. Notably for this review, both enadoline (0.0025–0.02 mg/kg) and ICI 204,448 (2.5–10 mg/kg) attenuated the scratching in a dose-related manner, giving antiscratch-50 values of 0.004 and 2.82 mg/kg, respectively (Cowan and Kehner 1997). Confidence in the pharmacological underpinnings of the Kuraishi model was reinforced by our observation of stereoselectivity for enadoline. Thus, as just noted, (−)-enadoline was active against compound 48/80-elicited scratching, whereas (+)-enadoline, which has very low affinity for kappa opioid receptors (Halfpenny et al. 1990), had no marked antiscratch effect (Kehner 2002) (Fig. 1 and Table 2). This result is in line with the comparable finding of stereoselective antagonism of bombesin-elicited scratching in rats by ethylketocyclazocine, the benzomorphan analgesic (vide supra).
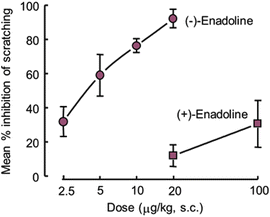

Fig. 1
Effects of (−)- and (+)-enadoline on scratching bouts in mice (n = 7–12). (−)-Enadoline (active enantiomer) dose-dependently antagonized scratching induced by compound 48/80 with an A50 value of 0.004 (0.002–0.005) mg/kg. (+)-Enadoline (essentially inactive enantiomer) had no marked antiscratch effect (A50 > 0.10 mg/kg). Both enantiomers were supplied by Parke-Davis, Cambridge, UK)
Table 2
Rank order of potency of opioid receptor ligands in suppressing repetitive scratching caused by compound 48/80 (2 mg/kg, s.c.) in micea
Compound | A50 (mg/kg, s.c.) |
|---|---|
(−)-Enadoline | 0.004 (0.002–0.005) |
Nalfurafine | 0.007 (0.004–0.009)b |
CR845 | 0.077 (0.035–0.24)c |
Asimadoline | 0.51 (0.28–0.76) |
U-50,488 | 1.34 (1.23–4.54) |
ICI 204,448 | 2.82 (1.38–4.26) |
ADL 10-0101 | 13.6 (7.4–19.7) |
(+)-Enadoline | >0.10 |
Naloxone | >3.0 |
norBNI | >20 |
Kappa receptors seem to be involved in this blunting of 48/80-evoked scratching since the behaviorally neutral dose of norBNI (20 mg/kg at −15 h) antagonized the antiscratch activity of a submaximal dose of either enadoline (0.01 mg/kg) or ICI 204,448 (5 mg/kg). Overall, these results strengthened the proposition that kappa agonists as a class are potential antipruritic agents. Moreover, the activity of ICI 204,448 (in contrast to the inactivity of this compound in the rat bombesin scratch model) established, for the first time, the possibility of developing peripherally directed kappa agonists as therapeutically important antipruritics. The hydrophilic ICI 204,448 has been a valuable pharmacological tool in basic animal studies on itch. For example, the standard dose (5 mg/kg) provides a stable antagonism (50–70 %) of compound 48/80-provoked scratching for as long as 2 h (when given as a pretreatment in different groups of mice) and then gradually fades over the following 3 h. A second example of the usefulness of a kappa agonist with low CNS penetration was our demonstration of antagonism of chloroquine-induced, quick onset scratching in mice by ICI 204,448, this finding clearly implicating peripheral kappa receptors in the compulsive behavior (Inan and Cowan 2004). Parenthetically, it may be of clinical interest to note that nalfurafine (vide infra), the centrally active kappa agonist, also suppressed this chloroquine-elicited, histamine-independent, frenzied scratching when administered (either subcutaneously or orally) to additional mice (Inan and Cowan 2004). Next, we conducted experiments with ICI 204,448 to address the following unanswered pharmacological question: does tolerance develop to the antiscratch activity of a peripherally restricted kappa agonist in the compound 48/80 model? Two studies were initiated with the acceptance that some ICI 204,448 would likely cross the blood–brain barrier under the multiple dosing schedules utilized. When ICI 204,448 (5 mg/kg, s.c.) was administered to mice every other day for 5 days (i.e., on days 1, 3, and 5), in parallel with appropriate control groups, there was no behavioral tolerance (Fig. 2, top panel). In contrast, when different groups of mice were similarly injected with 5 mg/kg of ICI 204,448 for 5 consecutive days, tolerance clearly developed (Fig. 2, bottom panel). So, as with all whole animal tolerance experiments, the conclusion is only meaningful when linked and discussed with the specific dosing schedule employed.
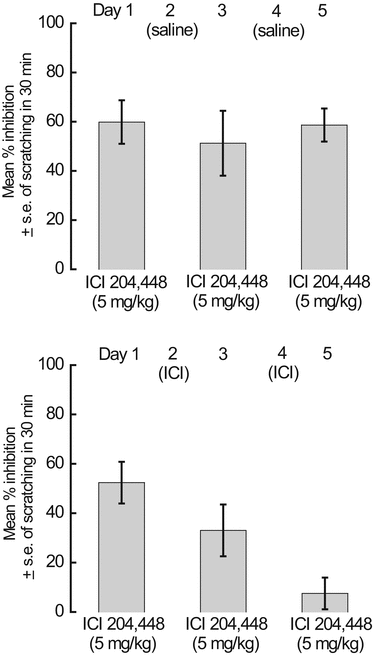

Fig. 2
The antiscratch effects of ICI 204,448 in the compound 48/80 mouse model were maintained after every-other-day injection of the kappa agonist for 5 days (top panel). In separate groups of mice, injected every day for 5 days, partial tolerance occurred on day 3, and full tolerance was demonstrated on day 5 (bottom panel). Each group represents the mean percent inhibition of scratching compared to controls (n = 7–12) (Kehner 2002)
In developing the chronology of the kappa antagonist-scratching story, it should be appreciated that parallel experiments with antihistamines also showed suppression of compound 48/80-evoked scratching. Thus, Sugimoto and colleagues (1998) called attention to histamine H-1 receptors by reporting that either chlorpheniramine or diphenhydramine antagonized subsequent scratching elicited by compound 48/80 (50 μg, s.c. in the nape of the neck) in BALB/c mice. More recent reports have stressed the prominent involvement of histamine H-4 receptors in mediating the compound 48/80 itch-associated response (e.g., Dunford et al. 2007). Structure–activity studies in the lowly mouse scratch test have encouraged further experimentation and, ultimately, demonstration of some efficacy for oral JNJ 39758979, a histamine H-4 antagonist, in attenuating histamine-induced pruritus in healthy (nonallergic) humans (Kollmeier et al. 2014).
4 Pruritogenic Activity of norBNI in Mice
Norbinaltorphimine (norBNI) is a bivalent ligand containing two naltrexone-derived pharmacophores (Fig. 3). Despite its description and widespread use as the prototype kappa receptor antagonist for almost three decades (Portoghese et al. 1987; McCurdy et al. 2006; Briggs and Rech 2009), the selectivity of norBNI (especially across the first hour or so after injection in rodents) has been questioned (Birch et al. 1987; Endoh et al. 1992; Spanagel et al. 1994). What is not in doubt is its long duration of action (a matter of weeks) as a kappa antagonist in mouse antinociceptive tests (Endoh et al. 1992; Horan et al. 1992; Broadbear et al. 1994). This long duration of action is a property shared by at least two other kappa opioid antagonists/pruritogens, JDTic and 5′-GNTI (vide infra) (Carroll et al. 2004; Bruchas et al. 2007). Explanations include activation of the c-Jun N-terminal kinase 1, mitogen-activated protein kinase cascade and, in consequence, inhibition of signaling at the kappa receptor (Bruchas et al. 2007; Melief et al. 2011), and long-term presence of compound (norBNI) in the central nervous system of mice (Kishioka et al. 2013; Patkar et al. 2013).
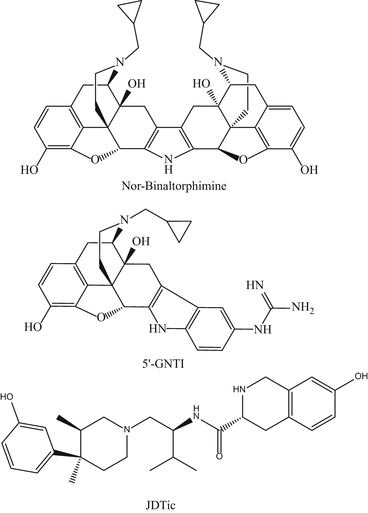

Fig. 3
Chemical structures of norbinaltorphimine, 5′-GNTI, and JDTic
All three compounds possess slow onsets of action as kappa antagonists (Broadbear et al. 1994; Carroll et al. 2004; Metcalf and Coop 2008). Neither attribute—delayed onset and long duration of action—was reported by Kamei and Nagase (2001) when they described, for the first time, the itch-associated properties of norBNI after subcutaneous injection (behind the neck) in ICR mice. Similarly, JDTic (0.30–5 mg/kg, s.c.), the phenylpiperidine-based kappa antagonist (Carroll and Carlezon 2013), caused quick onset scratching of very short duration in Swiss Webster mice (DiMattio, Liu-Chen, and Cowan, unpublished results).
The demonstration of repetitive scratching in mice by norBNI was notable for introducing a new class of pruritogen (and research tool) to the field of dermatopharmacology. Proposing an overall mechanism of action is no easy matter. norBNI caused no excessive scratching when given either icv to ddY mice (Umeuchi et al. 2003) or subcutaneously to rhesus monkeys (Ko et al. 2003). Kamei and Nagase (2001) reported that oral pretreatment with either chlorpheniramine or U-50,488 antagonized, in a dose-dependent manner, scratching precipitated by norBNI (10 mg/kg). On the basis of their interactional studies, these latter workers offered a conservative interpretation and speculated that the repetitive behavior was caused, in part, by (1) the release of histamine, followed by (2) antagonism of kappa opioid receptors.
5 Pruritogenic Activity of 5′-GNTI in Mice
We selected 5′-guanidinonaltrindole (5′-GNTI) for in-depth study as an alternative pruritogen to norBNI because of its superior potency and receptor selectivity as a kappa antagonist in standard smooth muscle preparations (Jones and Portoghese 2000; Stevens et al. 2000). Such was our thinking—associating kappa antagonism with body scratching—at the beginning of the 2000s (Cowan et al. 2002). Additionally, 5′-GNTI provided interesting structural novelty (Fig. 3), being the 5′-guanidinyl derivative of naltrindole the well-recognized nonpeptidic antagonist at delta opioid receptors (Portoghese et al. 1988, 1990).
5′-GNTI (0.03–3 mg/kg), but not naloxone (3 mg/kg), caused vigorous, compulsive scratching bouts within 3–5 min of subcutaneous injection in the nape of the neck of male Swiss Webster mice. 5′-GNTI was more efficacious and more potent (×44) than norBNI (Cowan and Inan 2009). A submaximal dose of 0.30 mg/kg of 5′-GNTI was chosen as standard for all experiments summarized below. Optimal scratching with this dose occurred between 10 and 30 min and faded gradually between 30 and 80 min. So, as with norBNI (Sect. 4), there is a temporal disconnect between the scratching phase and the time of peak effect of 5′-GNTI as a kappa antagonist (Munro et al. 2012). As of 2014, the display of scratching in mice as a consequence of kappa opioid antagonism (or not) represents a conundrum yet to be solved.
Scratching also occurred after intradermal injection of 5′-GNTI into the rostral back of mice but not when delivered by intraperitoneal, spinal (lumbar), or icv routes of administration. Sprague Dawley rats were essentially unaffected when given 5′-GNTI (0.30 or 3 mg/kg, s.c.) behind the neck, highlighting species as a variable in basic itch research. For context, Thomsen et al. (2001) reported dose-related scratching with serotonin in Sprague Dawley rats and characterized histamine, compound 48/80, and substance P as weak/inactive pruritogens in this strain.
The behavioral pharmacology of 5′-GNTI has been extended with the following observations (Inan et al. 2009a; Inan 2010; Cowan and Inan 2013): (1) tolerance to the scratch-inducing action of 5′-GNTI did not develop when mice were injected with the pruritogen once every day for 8 consecutive days, recalling a similar conclusion with bombesin in rats (Sect. 2); (2) the scratching was resistant to antagonism by naloxone (3 mg/kg, s.c.) or norBNI (20 mg/kg, i.p. at −18 h) but suppressed by intradermal 2 % lidocaine; (3) 5′-GNTI precipitated scratching in mu, kappa, or delta opioid knockout mice (C57BL/6 J background) to the same extent as in respective wild-type littermates; (4) oral pretreatment at −45 min with either a histamine H-1 antagonist (fexofenadine, 20–60 mg/kg) or a H-4 antagonist (JNJ 10191584, 10–60 mg/kg) proved ineffective; and (5) 5′-GNTI was more efficacious and potent (12.5×) than the 6′-regioisomer which, nevertheless, was a very active pruritogen in mice. Both 5′-GNTI and 6′-GNTI therefore exhibit a common overt behavior despite the latter compound being described as a selective agonist for the spinal kappa–delta heteromer (Van Rijn et al. 2010) and also as a biased agonist at kappa receptors (Rives et al. 2012).
Additionally, we investigated if gastrin-releasing peptide (GRP) might play a role in kappa antagonist-evoked scratching particularly in view of the report by Sun and Chen (2007) claiming that the spinal receptor for GRP mediates scratching in mice receiving chemically diverse pruritogens. We pretreated mice with RC-3095 (10 or 30 mg/kg, s.c.) in the flank (Inan et al. 2011). This pseudopeptide is a GRP (BB2) antagonist (Jensen et al. 2008; Andoh et al. 2011), which is bioavailable after systemic administration and which had no marked effect on the incidence of scratching associated with our standard dose of 5′-GNTI. We obtained similar negative results with [D-Phe6]bombesin(6–13)methyl ester (2–100 nmoles intraspinally), the peptide antagonist of GRP (BB2) receptors that was used previously by Sun and Chen (2007).
We submitted a sample of 5′-GNTI (Tocris Bioscience) to Caliper Life Sciences (now called PerkinElmer) for preliminary binding affinity screening at 1 μM against a panel of neurotransmitters, ion channels, steroids, second messengers, prostaglandins, growth factors, hormones, brain–gut peptides, and enzymes. Modest binding to muscarinic M-1 receptors was detected and subsequently confirmed by Munro et al. (2013). Our studies in mice involving interactions between 5′-GNTI and muscarinic M-1 agonists or antagonists are ongoing. A common interpretational problem in rodent behavioral research was encountered with intraspinal McN-A-343 (1.5–15 μg), the M-1 agonist: demonstration of statistically significant suppression of 5′-GNTI-evoked scratching (e.g., 70 %, p < 0.01 with 15 μg) despite the group mean from the 30 min observation session remaining impressively high (190 ± 35 [s.e.m.] scratches) (Inan et al. 2011).
So, as with many contemporary studies in the field of experimental pruritus, the following pesky question needs an answer: What is the pharmacological basis of the residual bouts of scratching often observed after incomplete antagonism of pruritogen-induced scratching, in this case, from pairing McN-A-343 with 5′-GNTI?
6 Behavioral Effects of Zyklophin in Mice
Zyklophin is a metabolically stable dynorphin analogue of 11 amino acids (Patkar et al. 2005). It is a selective kappa receptor antagonist that differs from the small molecule norBNI, 5′-GNTI, and JDTic, respectively, in being a cyclic peptide and in possessing a much shorter duration of action as a kappa antagonist (<12 h) after subcutaneous administration in the mouse tail withdrawal test (Aldrich et al. 2009). The peptide structure and shorter duration of action notwithstanding, zyklophin (0.10–1 mg/kg, s.c.) caused Swiss Webster mice to scratch their necks excessively within 1 min of injection. The extent of this behavior was dose related with 1 mg/kg of the peptide eliciting around 350 bouts in the 30 min observation period. Most of the scratching occurred within 15 min of injection and was essentially over by +30 min.
Pretreating mice with norBNI (20 mg/kg, i.p. at −18 h) had no marked influence on scratching associated with a submaximal dose of zyklophin (0.30 mg/kg). This result calls to mind the ineffectual antagonism of 5′-GNTI-evoked scratching by norBNI mentioned in Sect. 5. Similarities between zyklophin and 5′-GNTI were also apparent in their respective abilities to provoke scratching in kappa receptor knockout mice (C57BL/6 J background), comparable to that of wild-type C57BL/6 J controls (DiMattio et al. 2014; Sect. 5). This work unveiled an additional finding: male, wild-type C57BL/6 J mice are much less sensitive to a standard dose of zyklophin than are corresponding Swiss Webster animals. Still to be determined is the molecular target(s) at which zyklophin acts to elicit scratching.
7 Antipruritic Profile of Nalfurafine (TRK-820)
Nalfurafine holds pride of place in the history of kappa opioid pharmacology. Although identified initially as a prospective analgesic, this indication was never realized clinically and the compound was recast and developed as an antipruritic. Introduced as a kappa agonist by Nagase and colleagues in 1998, this 4,5-epoxy-morphinan represents the only marketed product to emerge from the active field of kappa opioid research. Nalfurafine (as Remitch® capsules) was launched in Japan in 2009 for the treatment of uremic pruritus in subjects with end-stage renal disease receiving hemodialysis and is currently a part of ongoing trials in the United States (uremic pruritus) and Japan (cholestatic itch).
Togashi et al. (2002) described the antiscratch activities of oral nalfurafine in ICR mice. Thus, the scratching invoked by either intradermally injected histamine or substance P was markedly suppressed by nalfurafine (0.10 mg/kg), and this action, in turn, was antagonized by norBNI, given subcutaneously at −24 h. The three main metabolites of nalfurafine were essentially inactive in the substance P itch model (Nakao et al. 2012). In our hands, nalfurafine was a very potent antagonist of either compound 48/80-induced scratching (Wang et al. 2005) or 5′-GNTI-induced scratching (Inan et al. 2009a) in Swiss Webster mice. In the latter study, nalfurafine was active when administered either before or after 5′-GNTI with doses that did not affect spontaneous locomotion. At the neuronal level, nalfurafine inhibited c-fos expression elicited by either 5′-GNTI or compound 48/80 in the dorsal horn of mouse spinal cord. This result supports a spinal locus of action for nalfurafine as an antiscratch agent (but not exclusively) against both pruritogens (Inan et al. 2009b).
Tolerance did not develop over 10 daily injections of nalfurafine (0.02 mg/kg, s.c.) to its antiscratch activity against 5′-GNTI. This finding was of translational value and contrasted with a previous report by Suzuki et al. (2004) stating that tolerance develops after only five administrations (over 3 days) to both the antinociceptive and sedative effects of nalfurafine in mice. As a pertinent footnote to this issue, tolerance did not occur to the well-known side effect—water diuresis—that accompanied daily injections of nalfurafine (0.02 mg/kg, s.c.) to rats for 7 days (Inan et al. 2009c).
The high selectivity of nalfurafine for the kappa opioid receptor over other opioid receptors was described by Wang et al. (2005). In routine screening for binding affinity at nonopioid receptors (quoted by Nakao and Mochizuki 2009), the profile for nalfurafine was unremarkable except for the detection of low binding affinity at muscarinic M-1 receptors, bringing to mind the same result with 5′-GNTI (Sect. 5). Nonetheless, this profile is sufficient to promote nalfurafine as a multi-target ligand with an increasingly wide spectrum of antiscratch activities. Here are two examples. First, in a rat model of scratching behavior secondary to cholestasis induced by chronic injections of ethynylestradiol, nalfurafine (0.005–0.04 mg/kg, s.c.) suppressed the whole-body scratching (suggestive of generalized pruritus) in a dose-dependent manner (Inan and Cowan 2006). And second, more recently, Akiyama et al. (2015) studied a mouse model of chronic dry skin itch (acetone/diethyl ether/water applied to the nape of each animal neck twice daily for 8 days) and found that systemically injected nalfurafine (0.02 mg/kg) abolished the usual spontaneous scratching.
First reports linking the beneficial effects of selective kappa agonists with pruritic states in humans date back to 2001 with a communication from Toray Industries on nalfurafine in uremic pruritus at the first meeting of the International Forum for the Study of Itch in Singapore, and a press release from Adolor Corporation on a successful proof-of-concept trial with ADL10-0101 against experimentally provoked poison ivy itch in adult volunteers. This latter compound was one of a series of peripherally directed arylacetamides that were active in animal models of itch (Table 2) and visceral pain. The itch project was subsequently terminated in favor of the pain project (Eisenach et al. 2003). With nalfurafine, several small, open-label studies led to the much quoted “Kappa opioid system in uremic pruritus: multicenter, randomized, double-blind, placebo-controlled clinical studies” by Wikström et al. (2005) in which belief in the safety and clinical potential of nalfurafine in alleviating pruritus in patients on hemodialysis was reinforced and eventually strengthened (Kumagai et al. 2010, 2012; Ueno et al. 2013). Mitsubishi Tanabe Pharma Corporation has partnered with Toray Industries to introduce MT-9938 (nalfurafine) into North America for treatment, in the first instance, of uremic pruritus in subjects with end-stage renal disease receiving hemodialysis.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


