Chapter 24 Miscellaneous isoprenoids
In addition to the groups of compounds considered in Chapters 22 and 23, there exists in nature a tremendous range of other isoprenoids, some of which have become of increasing interest as medicinal agents. There are also those plant metabolites of ‘mixed’ biogenetic origin which contain an isoprenoid moiety (e.g. some indole alkaloids, the cannabinoids and chlorophylls) and these are considered in other appropriate chapters.
MONOTERPENES
As illustrated in Figures 18.18 and 18.19, the monoterpenes are derived from the C10 geranyl pyrophosphate and constitute important components of volatile oils. Other examples are given below. Monoterpenoid compounds are reviewed regularly in Natural Product Reports (for coverage of the 1990 literature see D. H. Grayson, ibid., 1994, 11, 225).
IRIDOIDS
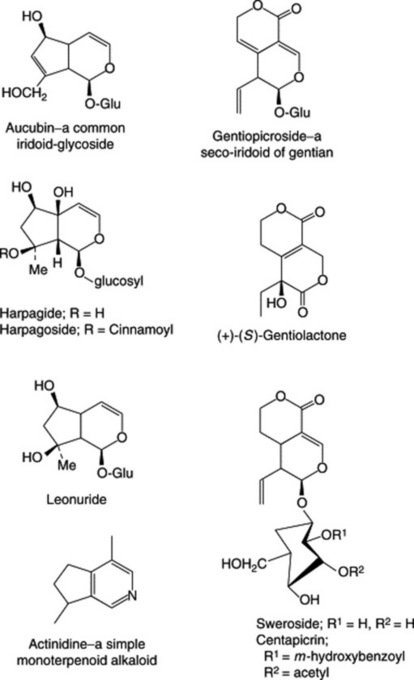
The iridoids are cyclopentan-[c]-pyran monoterpenoids and constitute a group of which the number of known members is constantly increasing. The name derives from Iridomyrmex, a genus of ants which produces these compounds as a defensive secretion. In a series of reviews covering the years up to December 1989 several hundred iridoids, classified originally into 10 groups, have been listed. Junior (Planta Med., 1990, 56, 1) has reviewed (146 refs) the isolation and structure elucidation of these compounds. Most occur as glycosides; some occur free and as bis compounds. There are many seco-iridoids, see secologanin, in which the pyran ring is open, and in a few the pyran ring oxygen is replaced by nitrogen, Fig. 24.1.
Of pharmaceutical significance is their presence in Valerian, Gentian and Harpagophytum and the involvement of loganin (Chapter 26) as a precursor of the non-indole portion of some alkaloids.
GENTIAN
Collection and preparation
When the plants are 2–5 years old, the turf is carefully stripped around each and the rhizomes and roots are dug up. This usually takes place from May to October, collection in the autumn being more difficult on account of the hardness of the soil, although possibly preferable from the medicinal point of view. There is no UK demand for ‘white’ or unfermented gentian, the commercialdrug consisting of ‘red’ or fermented gentian. The method of preparing this varies somewhat in different districts. Usually, the drug is made into heaps, which are allowed to lie on the hillside for some time and may even be covered with earth. After it is washed and cut into suitable lengths the drug is dried, first in the open air and then in sheds. Prepared in this way the drug becomes much darker in colour, loses some of its bitterness and acquires a very distinctive odour.
Constituents
The seco-iridoid gentiopicroside (also known as gentiopicrin and gentiamarin; formula see Fig. 24.1) is the principal constituent and was isolated from fresh gentian root in 1862. It occurs to the extent of about 2% and on hydrolysis yields a lactone (gentiogenin) and glucose. A biphenolic acid ester of gentiopicroside, amarogentin, which occurs in small amount (0.025 to 0.05%) has a bitterness value some 5000 times greater than that of gentiopicroside and is therefore an important constituent of the root; other bitters isolated are sweroside and swertiamarin. The isoprenoid gentiolactone has been separated into its enantiomers (Fig. 24.1) by HPLC involving a chiral column (R. Kakuda et al., Chem. Pharm. Bull., 2003, 51, 885) and the same group of workers (J. Toriumi et al., Chem. Pharm. Bull., 2003, 51, 89) has reported on new triterpenes in addition to α-amyrin, β-amyrin and lupeol.
The yellow colour of fermented gentian root is due to xanthones (Chapter 21) and includes gentisin (also known as gentiamarin) (Fig. 21.16), isogentisin and gentioside (a 3β-primeverosidoisogentisin). Gentian also contains gentisic acid (2,5-dihydroxybenzoic acid) and about 0.03% of the alkaloids gentianine and gentialutine, which may be artefacts of the preparation process.
Gentian is rich in sugars, which include the trisaccharide gentianose, the disaccharides gentiobiose and sucrose. During the fermentationprocess these are partially hydrolysed into glucose and fructose. If fermentation is allowed to proceed too far, the hexose sugars are converted into alcohol and carbon dioxide. Gentian should yield 33–40% of water-soluble extractive (BP not less than 33%), but highly fermented root yields much less.
CENTAURY
The drug has a very bitter taste due to small amounts of seco-iridoid glycosides. Compounds characterized include centapicrin, swertiamarin, sweroside and gentiopicroside. The BP TLC test for identity uses a swertiamarin/rutin test solution. Other constituents include flavonoids (up to 0.4%), methylated xanthone derivatives, traces of pyridine and actinidine alkaloids (Fig. 24.1), triterpenoids and various acids.
PLANTAIN
Constituents
The constituents appear similar for both species and include the iridoid aucubin and derivatives (Fig. 24.1), flavonoids, e.g. apigenin and luteolin (see Table 21.5), sugars, mucilage and various organic acids. The BP/EP requires a minimum of 1.5% of total o-dihydroxycinnamic acid derivatives expressed as acteoside and detects contamination of the drug with Digitalis lanata leaves by TLC.
VALERIAN ROOT
Valerian consists of the rhizome, stolons and roots of Valeriana officinalis L.s.l. (Valerianaceae), collected in the autumn and dried at a temperature below 40 °C. The plant is a perennial about 1–2 m high. It is obtained from wild and cultivated plants in The Netherlands, Belgium, France, Germany, eastern Europe and Japan. It is also cultivated in the USA. Polyploidy occurs in V. officinalis and there are diploid, tetraploid and octoploid types. British valerian is usually octoploid and central European usually tetraploid.
Constituents
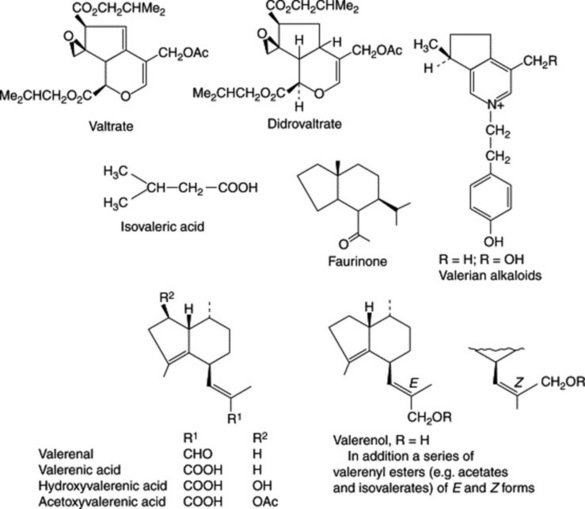
The drug yields about 0.5–1.0% of volatile oil. This contains esters (bornyl isovalerate, bornyl acetate (c. 13.0%), bornyl formate, eugenyl isovalerate, isoeugenyl isovalerate), alcohols, eugenol, terpenes and sesquiterpenes (e.g. valerenal, c. 12%). The latter comprise various acids, esters, alcohols and a ketone (faurinone) some of which are illustrated in the formulae shown (Fig. 24.2).
Seasonal variations in the constituents of valerian raised in the Netherlands have been reported. Thus the accumulation of valerenic acid and its derivatives together with valepotriates reached a maximum in February to March whereas the volatile oil remained essentially constant during the period of study. Strains producing 0.9% essential oil and a high content of valerenic acid and derivatives (0.5%) were recognizable (R. Bos et al., Planta Medica, 1998, 64, 143). For the clinical significance of such strains see ‘Action and uses’.
Allied drugs
Centranthus ruber root (Valerianaceae) also contains a number of the valepotriates of valerian.
DEVIL’S CLAW (HARPAGOPHYTUM)
The plant, which derives its name from the characteristic structure of the fruit, is native to Southern and Eastern Africa and is largely obtained from Namibia, with lesser amounts from S. Africa and Botswana. In 2002, at the height of the drug’s popularity, exports from S. Africa amounted to some 1018 tonnes of dried tubers, representing millions of plants. To avoid extinction of the plant, a proposal was made to add it to the CITES list but in deference to the effect on the economy of rural areas this was withdrawn and efforts were initiated to develop microprogation techniques to solve the problem. For a full review, see ‘Further reading’.