Chapter 6 Isomers
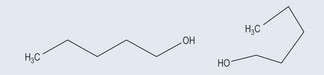
If two compounds have the same molecular formulae (in other words, the same number and types of atoms) but the atoms are arranged differently, they are classed as isomers. Care has to be taken with this definition, however, as the two examples in Figure 6.1 are not isomers but the same molecule bent into a different shape because the mobility that the single bond allows it. In this example, the arrangement of atoms differs by their arrangement in space only.
Bear in mind that a single bond enables a molecule to rotate freely around it, so the apparent change in the shape in the molecule does not make it an isomer (compare this with the limited rotation around a double bond, see Figure 6.5).
There are several different types of isomer:
Structural Isomers
Chain isomerism
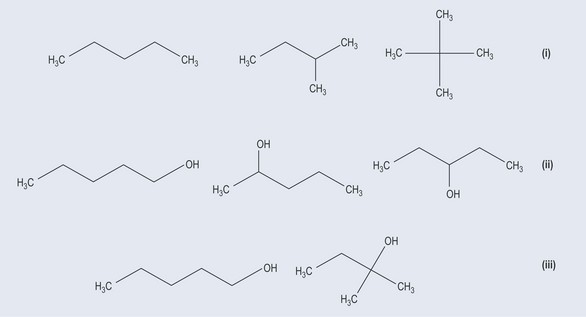
Chain isomers (Figure 6.2(i)) occur because functional groups can branch off from the main carbon backbone. The number of carbon and hydrogen atoms remains the same but the structures are very different.
Positional Isomerism
In positional isomers (Figure 6.2(ii)) the structure of the carbon backbone remains the same but the functional groups or side chains are moved around the backbone.
Stereoisomers
Entaniomers
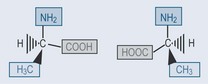
Enantiomers are stereoisomers that are mirror images of each other and all thecomponents of the compound radiating from one point are different. The term forthis is chiral. The position of the compound around which this occurs is a chiral centre (see Figures 6.3, and Figures 6.7). Take, for example, natural alanine, which is foundin only one isomeric form: L -alanine ( Figures 6.3). If alanine is produced synthetically, then both forms L and D alanine, are found. This is signifi cant when it comes togiving a patient nutritional supplementation, as the L isomer is more likely to fit intothe receptor site of an enzyme, which explains why natural products are favoured innutritional supplementation.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree