19 Ion channels
Voltage-gated cation channels
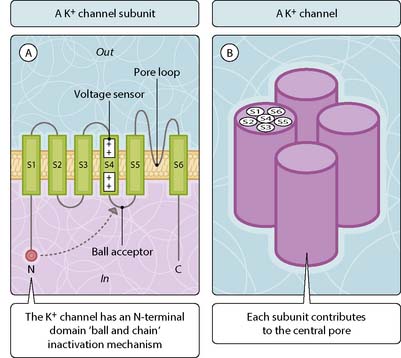
Voltage-gated (also known as voltage-operated or voltage-sensitive) cation channels share a common basic design based on a core structure of six hydrophobic membrane-spanning segments (S1–S6) (Fig. 3.19.1). The loop-linking segments S5 and S6 (H5) folds back into the membrane as a hairpin loop to form the pore lining. Four structural units are required to constitute a channel pore. Voltage sensitivity is conferred by the S4 transmembrane segment (voltage sensor), which contains a positively charged residue (lysine or arginine) at every third position in the primary sequence. The outward movement of this segment in response to the electrical force imposed by membrane depolarization is thought to drive the opening of voltage-gated channels. In those voltage-gated channels that inactivate rapidly, an inactivation ‘ball’ swings into a vestibule ‘ball acceptor’ domain on channel activation to block the pore. This ball is derived from the N-terminal region in these K+ channels (N-type inactivation). A second relatively rapid form of inactivation (C-type) has been characterized in some K+ channels. In Na+ and Ca2+ channels, which comprise four linked repeating channel units, cytoplasmic loops rather than the N-terminal domain form the ball.