Fig. 1.1
Drug discovery and development process
1.1.2 Chemistry, Manufacturing and Control (CMC)
After a lead compound is discovered and moves through different development stages, a supply of the compound is required for a sequence of testing in vitro, in vivo and in humans to establish its safety and efficacy profiles. The scale and quality standard of drug supply varies at different stages, and accordingly, the manufacturing processes and analytical methods also evolve. In the following, we will discuss drug manufacturing and quality testing at different stages from discovery to submission and post approval, using small molecules as an example.
At the discovery stage, after a disease target is identified, high throughput screening assays are used to generate hits— molecules that interact with the target in the desirable way. The physiochemical properties of the best “hit” compounds, sometimes called leads, are then tested with different assays. Cardiotoxicity and hepatotoxicity will also be tested before a compound moves to the preclinical stage. In these assays at the discovery stage, the amount of each compound required is usually at a milligram level for small molecules. Drug supply at this level can be synthesized by discovery scientists in a flask. Purity of the synthesized drug is characterized but impurities are not usually identified or quantified at this stage.
At the preclinical stage, toxicity and other safety studies require a significantly larger amount of drug, from a scale of grams to hundreds of grams. The production of such drug supply is transferred to a department in the Research and Development (R&D) organization, responsible for manufacturing active pharmaceutical ingredients (APIs). A manufacturing process is developed, along with analytical methods to characterize the API and to identify and quantify impurities. Raw materials are sourced with appropriate quality control. Sometimes outcome of safety studies and drug metabolism tests may indicate a heightened concern for safety or bioavailability, leading to a modification to the synthesization, an optimization of manufacture process and analytical methods, or a re-sourcing of some raw materials. Figure 1.2 includes a typical flowchart of API manufacture process (the part with dashed lines).
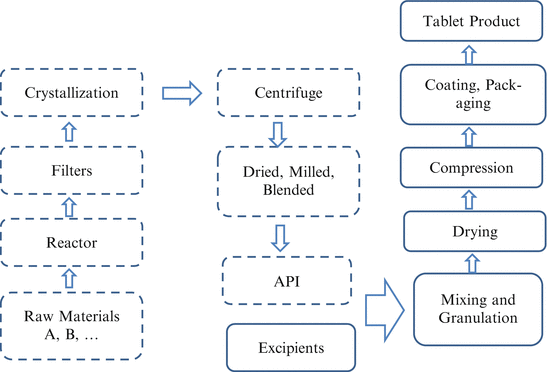

Fig. 1.2
Drug manufacturing and tableting process
After the IND, the compound can be tested in clinical trials. Drug supply for testing on human beings is required to follow current good manufacturing practice (cGMP), which is enforced by the FDA and provides for systems that assure proper design, monitoring and control of manufacturing processes and facilities. Medicines manufactured following cGMP are expected to meet desired quality standards. For most small molecule drugs, drug supply for early phase trials is usually in the form of a simple formulation of API, for example, capsules. However, they are typically in the form of tablets for final drug product. Before confirmatory trials, a formulation of API has been decided and a manufacturing process and analytical methods have been developed, optimized and validated. These trials typically require kilograms or more of the drug products, at least three batches to monitor the robustness of the process and analytical methods. Some of the drugs will be put on stability study at the proposed storage condition (typically room temperature). The data will be used to project a shelf life based on critical quality attributes (for example, potency and impurity) and corresponding specifications (ICH Q1). Figure 1.2 includes a typical tableting process (the part with solid lines).
Since the efficacy and safety of the drug confirmed in these trials is conditional on the quality of drug used, which in turn is conditional on the manufacturing processes and analytical methods, the commercial scale manufacturing after the drug is approved for marketing should use the same manufacturing process and analytical methods as for the confirmatory clinical trials. Any change to the process or analytical methods needs to show improvement or comparability to those used for registration trials.
1.1.3 Differences Between Small and Large Molecules
The discussion in Sects. 1.1.1 and 1.1.2 focuses on small molecules. Another type of therapeutics is large molecules or biologics that requires some differences in terms of discovery, development and manufacture. Small molecules are usually chemical compounds that have molecular weight less than 500 Da, are produced by chemical synthesization and often administered orally in the formulation of tablets, are metabolized by liver and gut, enter systemic circulation through gut wall, enter all sites of the body by penetrating cell membranes, and have a short half-life. Large molecules are peptides or proteins that have molecular weight more than 500 Da, are produced by recombinant DNA technology and cell culture, are formulated in lyophilized or liquid format and administered through injection (otherwise, reduced by stomach before reaching systemic circulation), cannot penetrate cell membranes, and have a longer half-life. For detailed difference between these two types of therapeutics, refer to Samanen (2013). The differences historically distinguished traditional pharmaceutical and biotechnology companies in that the former focused on small molecule drugs while the latter focused on large molecule drugs. However, now most major pharmaceutical companies have both small and large molecules in their product portfolios.
1.2 Definition of Nonclinical Statistics
Since this book is written about nonclinical statistics, the first question is, “what is nonclinical statistics?” To answer this question, we will start with the definition of statistics. According to Dictionary.com, “Statistics is the science that deals with the collection, classification, analysis, and interpretation of numerical facts or data, and that, by use of mathematical theories of probability, imposes order and regularity on aggregates of more or less disparate elements.” Statistical science has found applications in many fields, leading to branches with distinct names. For example, ecomometrics is statistics applied to economics, psychometrics is statistics applied to psychology, and actuary is statistics applied to life insurance, and so on.
Biostatistics is the application of statistics in fields related to biology, including medicine, pharmacy, agriculture, and fishery. Obviously, clinical biostatistics, statistics that supports clinical trials, is part of biostatistics. In fact, to many people in pharma/biotech industries, clinical biostatistics and biostatistics are interchangeable, due to the fact that in most universities departments of Biostatistics are located in medical schools and the emphasis of training is on statistical methods for clinical trials.
Clinical biostatistics seems to be a mature discipline. There is an academic department of this discipline in many universities. There are numerous journals dedicated to the subject, such as, statistics in medicine, journal of biopharmaceutical statistics, statistics in biopharmaceutical research, biometrics, biometrical journal, etc. Online searches reveal a huge collection of books dedicated to clinical biostatistics. In pharmaceutical or biotechnology companies, there is usually a department of biostatistics, organizationally located in clinical development in most companies, comprising mostly clinical statisticians. Clinical statisticians, as regulated in ICH E6 and E9, participate in design, monitoring, data analysis and reporting, and final result interpretation of clinical trials. They are a key member in clinical project teams, along with clinicians or physicians. As Lendrem (Lendrem 2002) commented, this is a “shining example of how an industry has embraced statistics and statisticians as equal partners in a joint venture.”
In contrast, the story for nonclinical statistics is quite different. First, there is not such a department in many companies. Instead, there is usually just a functional group. Second, the group name is not universal. According to a 2008 nonclinical statistics leadership survey (referred to as Survey 2008 in the following), the group name includes “pharmaceutical sciences statistics”, “Preclinical and Development Statistics”, “Biometrics Research”, “Statistical Services”, “Research&Translational Sciences Statistics”, “Preclinical and Research Biostatistics”, with “Nonclinical Statistics” as the most common one. These names reveal what scientific functions the nonclinical statistics group supports. For example, Preclinical and Research Biostatistics may support preclinical development and discovery, whereas Preclinical and Development Statistics could support preclinical development and CMC. We will discuss why we prefer “Nonclinical Statistics” shortly. Third, there are a much smaller number of nonclinical statisticians compared to clinical statisticians. More details on this come later. Lastly, nonclinical statisticians usually do not have a formal role in any project team. More often, they serve as internal consultants.
In spite of the divergence, we try to give a definition for “Nonclinical Statistics.” We define “Nonclinical Statistics” as statistics applied to areas other than clinical trials in pharmaceutical/biotechnology industries; these areas primarily comprise discovery, nonclinical development/translational science and CMC. Note that we use nonclinical development instead of preclinical development because the former concerns all safety studies after a compound’s discovery, whereas the latter only includes safety studies prior to clinical trials. After the IND and first in human milestones, and parallel to clinical development, many safety studies are still conducted, such as carcinogenicity studies, long term toxicity studies. Similarly CMC includes API and drug product development as well as corresponding analytical method development and validation in parallel to clinical development. The drug supply from CMC needs to nonclinical development, clinical trial materials and commercial marketing. We also extend this definition to include translational sciences where many biomarker studies take place in clinical trials.
< div class='tao-gold-member'>
Only gold members can continue reading. Log In or Register to continue
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


