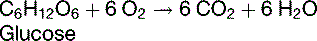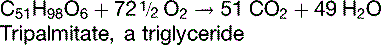Chapter 20 Introduction to Metabolic Pathways
The metabolic activities of cells are dictated by two major concerns:
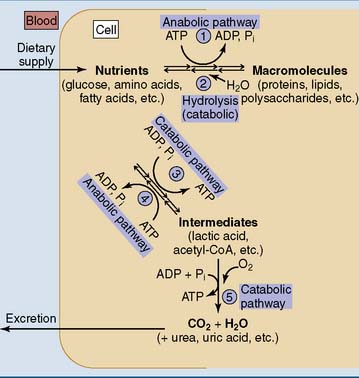
 in Fig. 20.1).
in Fig. 20.1).The biosynthesis of cellular macromolecules has to be balanced by their degradation (pathway  in Fig. 20.1). Under steady-state conditions, the rates of synthesis and degradation are balanced and the amount of the macromolecule remains constant. Different nutrients can also be interconverted through metabolic intermediates. For example, most amino acids can be converted to glucose, and glucose can be converted to amino acids and fatty acids (pathways
in Fig. 20.1). Under steady-state conditions, the rates of synthesis and degradation are balanced and the amount of the macromolecule remains constant. Different nutrients can also be interconverted through metabolic intermediates. For example, most amino acids can be converted to glucose, and glucose can be converted to amino acids and fatty acids (pathways  and
and  in Fig. 20.1).
in Fig. 20.1).
Alternative substrates can be oxidized in the body
The RQ for the oxidation of carbohydrates is 1.0; for fat, about 0.7; and for protein, about 0.8.
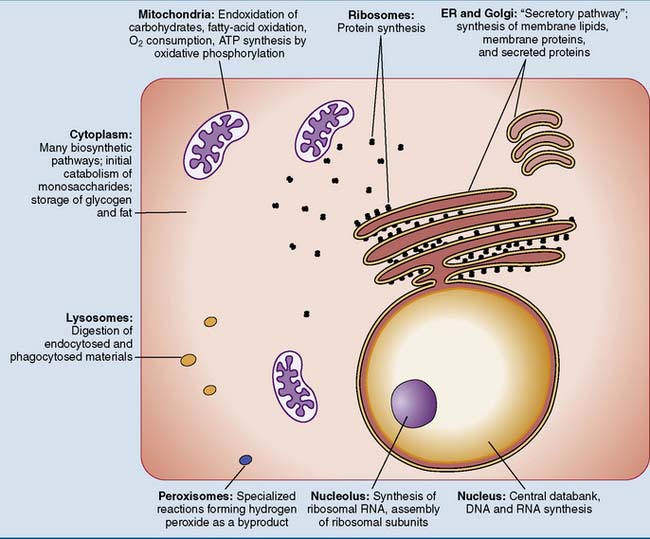
Metabolic processes are compartmentalized
In the cell, metabolic processes are compartmentalized. Each organelle has its own enzymatic outfit and metabolic activities (Fig. 20.2).
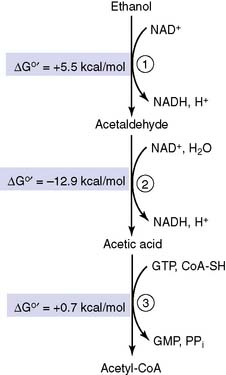
Free energy changes in metabolic pathways are additive
However, the actual free energy change is quite different from the standard free energy change. For example, reaction (1) has a very unfavorable equilibrium. With equal concentrations of NAD+ and NADH, there would be only one molecule of acetaldehyde at equilibrium for every 10,000 molecules of ethanol (see Chapter 4)! However, under aerobic conditions, [NAD+] is always far higher than [NADH]. When NAD+
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree