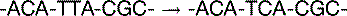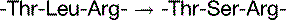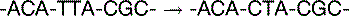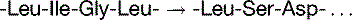Chapter 9 Introduction to Genetic Diseases
Mutations are an important cause of poor health
Children are, on average, a little sicker than their parents because they have new mutations on top of those inherited from the parents (see CLINICAL EXAMPLE 9.1). This mutational load is kept in check by a form of natural selection called purifying selection. In most traditional societies, almost half of all children died before they had a chance to reproduce. We can only guess that those who died had, on average, more “mildly detrimental” mutations than those who survived. Therefore the prevalence of disease-promoting mutations, and of the diseases themselves, is determined in large part by mutation-selection balance.
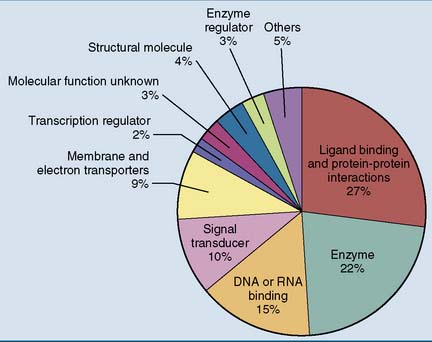
Four types of genetic disease
Four types of genetic disease are commonly distinguished.
The basal mutation rate is caused mainly by replication errors
The basal mutation rate, which is observed in the absence of environmental mutagens, is caused mainly by errors during DNA replication. These replication errors have an important consequence. Because the number of mitotic divisions before formation of the gametes is far greater for spermatogonia than oogonia, most base substitutions and small insertions and deletions originate in the paternal rather than the maternal germ line (see Clinical Example 9.1).
Similarly, adenine has a rare imino form that pairs with cytosine rather than thymine (Fig. 9.3). Fortunately, these bases spend very little time in their less stable forms; thus, mutations caused by tautomeric shifts are rare.
Mutations can be induced by radiation and chemicals
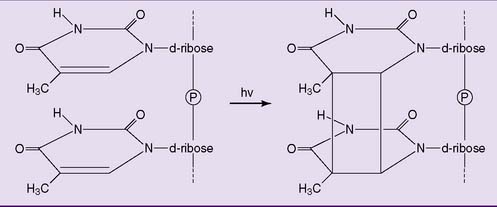
Ultraviolet radiation is a mutagenic component of sunlight. It cannot penetrate beyond the outer layers of the skin and therefore is unable to cause germline mutations. It only causes sunburn and skin cancer, mainly through the formation of pyrimidine dimers (Fig. 9.4).
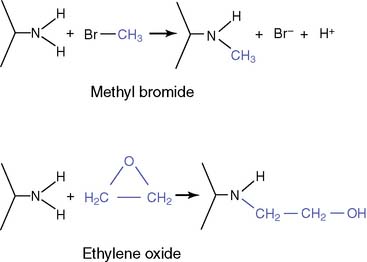
Many chemicals can act as mutagens.
Methyl bromide was used as a grain fumigant before it was banned for this use because of its carcinogenic properties. Ethylene oxide is used for the sterilization of surgical instruments. Nonenzymatic methylation by S-adenosyl methionine (SAM, see Chapter 5) is an important endogenous source of methylated bases. About 4000 7-methylguanosine, 600 3-methyladenine, and 10 to 30 O6-methylguanosine residues are formed by SAM in each cell per day.
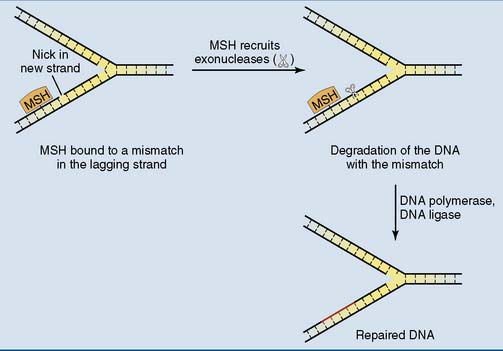
Mismatch repair corrects replication errors
The bound repair proteins recruit exonucleases to the nick, which then remove the DNA of the new strand between the nick and the mismatch, including the mismatch itself. This sets the stage for DNA polymerase δ and DNA ligase to fill the gap and connect the loose ends (Fig. 9.7). This system is most important for rapidly dividing cells (Clinical Example 9.2).