Chapter 1 Introduction to Biomolecules
Biochemistry is concerned with the molecular workings of the body, and the first question we must ask is about the molecular composition of the normal human body. Table 1.1 lists the approximate composition of the proverbial 75-kg textbook adult. Next to water, proteins and triglycerides are most abundant. Triglyceride (aka fat) is the major storage form of metabolic energy and is found mainly in adipose tissue. Proteins are of more general importance. They are major elements of cell structures and are responsible for enzymatic catalysis and virtually all cellular functions. Carbohydrates, in the form of glucose and the storage polysaccharide glycogen, are substrates for energy metabolism, but they also are covalently linked components of glycoproteins and glycolipids. Soluble inorganic salts are present in all intracellular and extracellular fluids, and insoluble salts, most of them related to calcium phosphate, give strength and rigidity to human bones.
Table 1.1 Approximate Composition of a 75-Kg Adult
| Substance | Content (%) | |
|---|---|---|
| Water | 60 | |
| Inorganic salt, soluble | 0.7 | |
| Inorganic salt, insoluble* | 5.5 | |
| Protein | 16 | |
| Triglyceride (fat)† | 13 | |
| Membrane lipids | 2.5 | |
| Carbohydrates | 1.5 | |
| Nucleic acids | 0.2 |
Water is the solvent of life
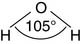
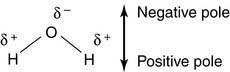
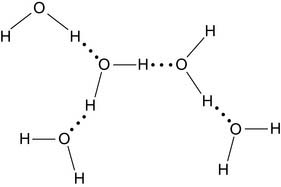
Charles Darwin speculated that life originated in a warm little pond. Perhaps it really was a big warm ocean, but one thing is certain: We are appallingly watery creatures. Almost two thirds of the adult human body is water (see Table 1.1). The structure of water is simplicity itself, with two hydrogen atoms bonded to an oxygen atom at an angle of 105 degrees:
These hydrogen bonds are weak. Only 29 kJ (7 kcal) per mole is needed to break a hydrogen bond in water, whereas 450 kJ (110 kcal) per mole* is required to break a covalent oxygen-hydrogen bond in the water molecule itself. Breaking the hydrogen bonds requires no more than heating the water to 100°C. The hydrogen bonds determine the physical properties of water, including its boiling point.
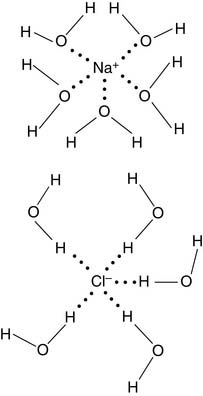
The water in the human body always contains inorganic cations (positively charged ions), such as sodium and potassium, and anions (negatively charged ions), such as chloride and phosphate. Table 1.2 lists the typical ionic compositions of intracellular (cytoplasmic) and extracellular (interstitial) fluid. Interestingly, the extracellular fluid has an ionic composition similar to seawater. We carry a warm little pond with us, to provide our cells with their ancestral environment.
Table 1.2 Typical Ionic Compositions of Extracellular (Interstitial) and Intracellular (Cytoplasmic) Fluids
| Concentration (mmol/L) | ||
|---|---|---|
| Ion | Extracellular Fluid | Cytoplasm |
| Na+ | 137 | 10 |
| K+ | 4.7 | 141 |
| Ca2+ | 2.4 | 10–4* |
| Mg2+ | 1.4 | 31 |
| Cl– | 113 | 4 |
| HPO42–/H2PO4– | 2 | 11 |
| HCO3– | 28† | 10† |
| Organic acids, phosphate esters | 1.8 | 100 |
| pH | 7.4 | 6.5–7.5 |
* Cytoplasmic concentration. Concentrations in mitochondria and endoplasmic reticulum are much higher.
† The lower HCO3– concentration in the intracellular space is caused by the lower intracellular pH, which affects the equilibrium:
Water contains hydronium ions and hydroxyl ions
Water molecules dissociate reversibly into hydroxyl ions and hydronium ions:
In pure water, only about one in 280 million molecules is in the H3O+ or OH– form:
With Equations (3) and (4), the H+ and OH– concentrations can be predicted at any given pH value (Table 1.3).
Table 1.3 Relationship among pH, [H+], and [OH–]
| pH | [H+]* | [OH–]* |
|---|---|---|
| 4 | 10−4 | 10–10 |
| 5 | 10−5 | 10–9 |
| 6 | 10–6 | 10–8 |
| 7 | 10–7 | 10–7 |
| 8 | 10–8 | 10–6 |
| 9 | 10–9 | 10–5 |
| 10 | 10–10 | 10–4 |
* [H+] and [OH–] are measured in mol/L (M).
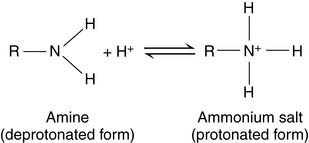
Ionizable groups are characterized by their pK values
the dissociation constant KD is defined as
The molar concentrations in this equation are the concentrations observed at equilibrium. Because the hydrogen ion concentration [H+] is most conveniently expressed as the pH value, Equation (6) can be transformed into the negative logarithm:
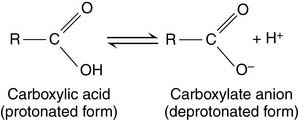
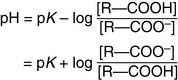
In the Henderson-Hasselbalch equation, pK is a constant, whereas [R—COOH]/[R—COO−] changes with the pH. When the pH value equals the pK value, log[R—COOH]/[R—COO−] must equal zero. Therefore [R—COOH]/[R—COO−] must equal one: The pK value indicates the pH value at which the ionizable group is half-protonated. At pH values below their pK (i.e., high [H+] or high acidity), ionizable groups are mainly protonated. At pH values above their pK (i.e., low [H+] or high alkalinity), ionizable groups are mainly deprotonated (Table 1.4)
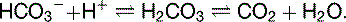
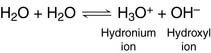 (1)
(1)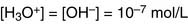 (2)
(2)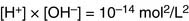 (3)
(3)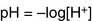 (4)
(4)
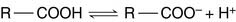
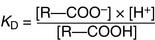 (5)
(5)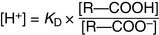 (6)
(6) (7)
(7)