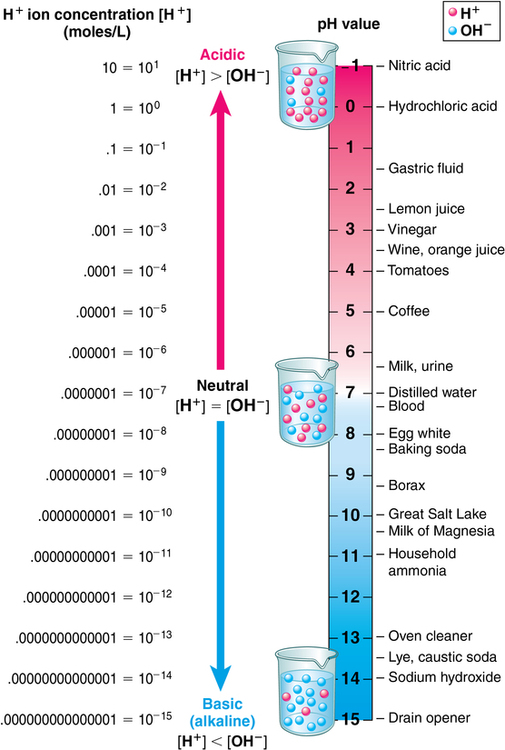
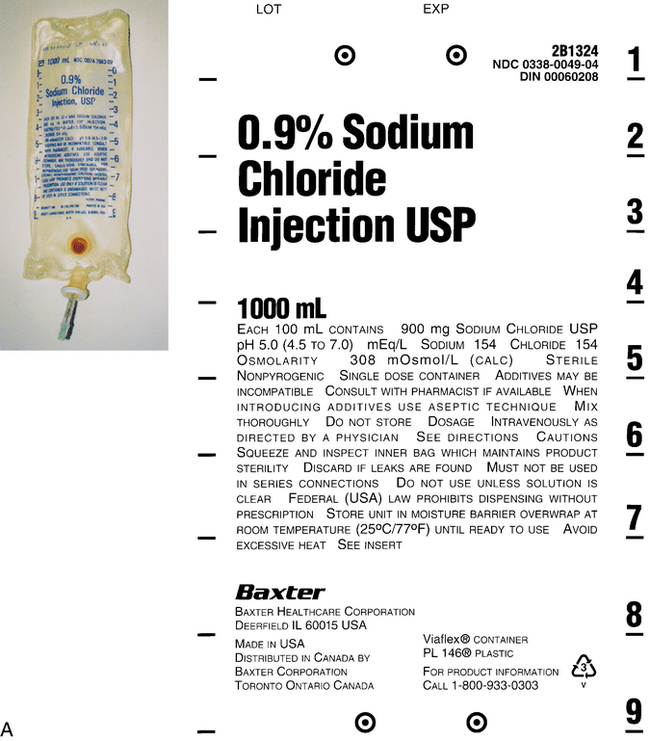
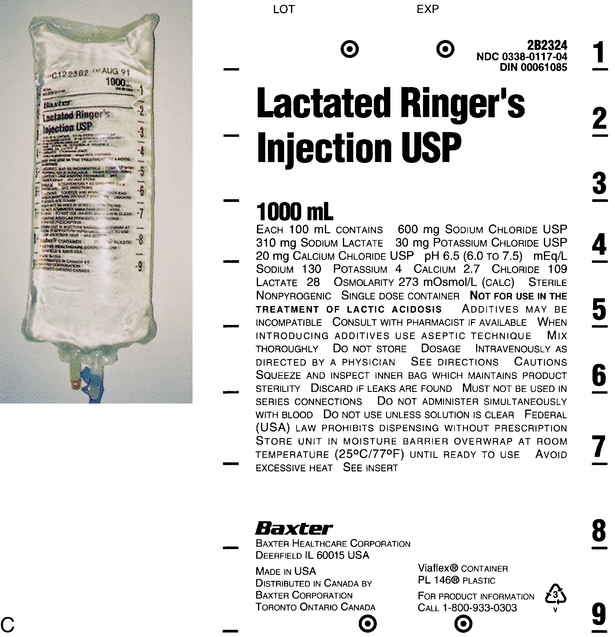
1. Discuss common intravenous fluids, including the abbreviations used. 2. Explain visual inspection of a parenteral solution. 3. List several factors that affect compatibility and stability of an intravenous solution. 4. Use reference materials to find incompatibility, compatibility, and storage information. The preparation of an intravenous (IV) medication that requires a mixture of medications Clear and free of visible particulate matter Ability to combine drugs or substances without interfering with their action Any solution containing a higher concentration of dissolved substances than red blood cells Any solution containing a concentration of dissolved substances less than red blood cells Number of dissolved particles in a solution per liter of solution Movement of a solvent (water) across a cell membrane from a lower osmolality to a higher osmolality Process of adding a diluent to a powder form of a medication IV solutions used in parenteral administration must be prepared using aseptic technique and have certain characteristics. They must be sterile (that is, free from bacteria). They must also have clarity, which means clear and free of visible particulate matter. In addition to the clarity and being bacteria free, the solutions also have characteristics that determine how they will act once they are in the blood stream. Osmosis is the passage of water particles from an area of lower concentration to an area of higher concentration across a barrier, such as a cell membrane. The number of these dissolved particles per liter of solution is known as osmolarity. The pH of the solution is also very important because the body’s fluid is slightly alkaline or about 7.4 (Figure 4-1). Fluid entering the bloodstream that is too acidic or too alkaline can cause pain or discomfort, and damage to the red blood cells should be avoided if possible. The fluid inside the cell contains dissolved substances, such as sugars and salts. The cell membrane is designed to allow fluid to pass freely from one membrane to another but not the substances. Isotonic fluids have the same osmolarity as normal body fluid. These solutions are the closest to the red blood cells because the concentration of the salt and other substances are the same as those found in red blood cells. Both 5% dextrose and 0.9% sodium chloride are examples of isotonic fluids. If the solution contains a concentration of dissolved substances less than red blood cells, it is known as hypotonic. This means fluid will move into the cells and cause swelling. If the solution contains a higher concentration than the red blood cells, it is hypertonic and cells can shrink due the movement of fluids out of the cells. Both of these types of fluids can cause stinging because the cells are trying to either swell or shrink to handle the fluids introduced (Figure 4-2). There are several common IV fluids available today. Medications that are added to the fluid are known as additives, whereas the final product is known as the admixture. Common IV fluids include sodium chloride injection, dextrose injection, and lactated Ringer’s solution for injection (Figure 4-3). Dextrose injection (glucose) is primarily used as a carbohydrate for nutrition and as a source of fluid. It is usually given in 5% concentration. Sodium chloride is used as a source of fluid and electrolytes. It is usually given in 0.9%. Lactated Ringer’s solution for injection contains primary electrolytes found in plasma and is used for fluid replacement or as a source of electrolytes. All of these fluids can be found in various combinations and can also be manufactured with certain additives already in them. There are also standard abbreviations that are used when writing orders or prescriptions that technicians should be familiar with (Table 4-1). TABLE 4-1 Standard Abbreviations and Tonicity of Intravenous Solutions
Intravenous solutions, stability, and incompatibilities
Introduction
Characteristics of different solutions used in intravenous therapy


IV Solution
Abbreviation
Tonicity
5% Dextrose
D5W
Isotonic
0.9% Sodium chloride
NS (normal saline)
Isotonic
Lactated Ringer’s solution
LR
Isotonic
10% Dextrose
D10W
Hypertonic
0.45% Sodium chloride (normal saline)
1/2NS
Hypotonic
5% Dextrose and 0.9% sodium chloride
D5NS
Hypertonic
5% Dextrose and 0.45% sodium chloride
D51/2NS
Hypertonic
5% Dextrose and 0.2% sodium chloride
D51/4NS
Isotonic
Lactated Ringer’s solution and 5% dextrose
D5LR
Hypertonic ![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Intravenous solutions, stability, and incompatibilities
