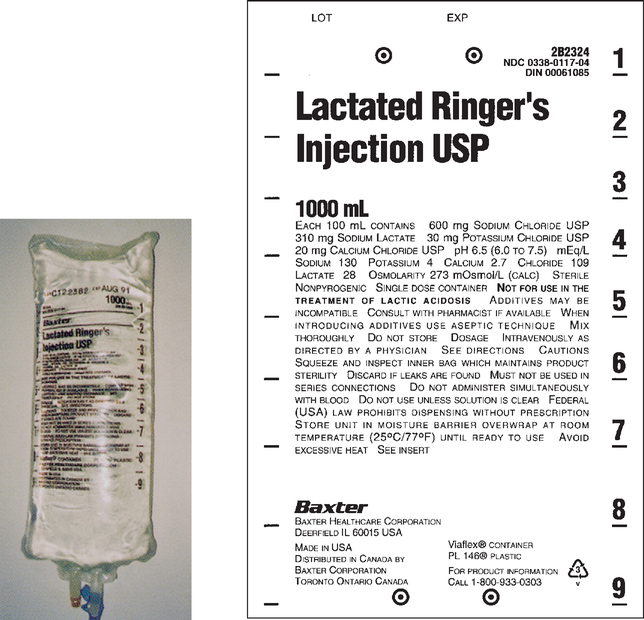
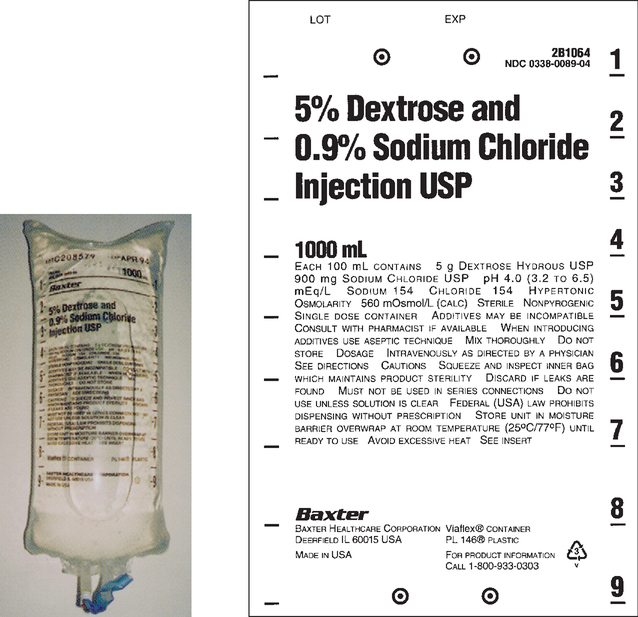
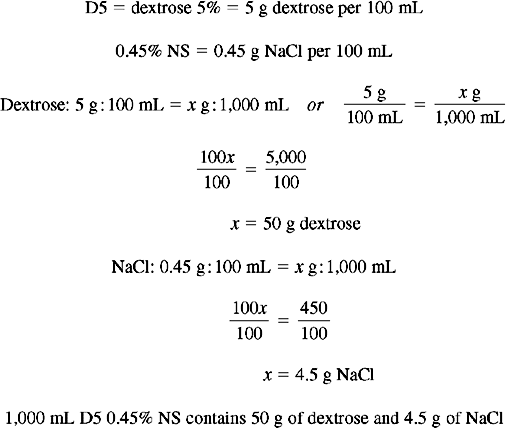CHAPTER 21 After reviewing this chapter, you should be able to: 1. Identify common intravenous (IV) solutions and abbreviations 2. Calculate the amount of specific components in IV solutions 3. Define the following terms associated with IV therapy: peripheral line, central line, primary line, secondary line, saline/heparin locks, IV piggyback (IVPB), and IV push 4. Differentiate among various devices used to administer IV solutions (e.g., patient-controlled analgesia [PCA] pumps, syringe pumps, volumetric pumps) The calculations associated with IV therapy will be discussed in the next chapter (Chapter 22). A general discussion of IV therapy will make it easier to understand the calculations that are performed involving IV therapy. Intravenous (IV) means the administration of fluids or medications through a vein. Medications, electrolyte solutions, and blood and blood products are frequently ordered and administered directly into the vein. The advantage of administering medications by this route is the immediate availability of the medication to the body and the rapidity of action. IV fluids are ordered for varied reasons. They may be ordered to restore or maintain fluid and electrolyte balance, to replace lost fluids, and to act as a medium for administering medications directly into the bloodstream. Replacement fluids are ordered for a client who has lost fluids because of things such as diarrhea, vomiting, or hemorrhage. Maintenance fluids help to sustain normal levels of fluids and electrolytes for clients at risk for depletion (e.g., a client NPO). Calculations related to IV therapy may include calculating infusion rates in drops per minute (gtt/min) or milliliters per hour (mL/hr). The calculations necessary for the safe administration of IV fluids will be presented in Chapter 22. The prescriber is responsible for writing the IV order, which must specify the following: For example, an IV order might read: There are several types of IV fluids. The type of fluid used is individualized according to the client and the reason for its use. IV solutions come prepared in plastic bags or glass bottles ranging from 50 mL (bags only) to 1,000 mL. IV plastic bags are more commonly used. IV solutions are clearly labeled with the exact components and amount of solution. When IV solutions are written in orders and charts, abbreviations are used. Example: D5W means dextrose 5% in water. You may encounter various abbreviations; however, the percentage and initials, regardless of how they are written, have the same meaning: “D” is for dextrose, “W” is for water, “S” is for saline, and “NS” is for normal saline. Ringer lactate (lactated Ringer), a commonly used electrolyte solution, is abbreviated “RL” or “LR” and occasionally “RLS.” Potassium chloride (KCl) is commonly added to IV solutions. Potassium chloride is measured in milliequivalents (mEq). The order is usually written to indicate the amount of milliequivalents per liter (1,000 mL) to be added to the IV fluid. Example: 0.9 % NS 1,000 mL IV with 20 mEq KCl per L q8h; this is interpreted as “infuse 1,000 mL 0.9 % normal saline IV solution with 20 milliequivalents potassium chloride added per liter every 8 hours.” Saline (sodium chloride or NaCl) is also found in IV fluids. Refer to Box 21-1, and learn the common abbreviations for IV solutions. Figures 21-1 to 21-5 show various IV solutions. Other saline solution strengths include 0.33% NaCl, also abbreviated as “1/3 NS,” and 0.225% NaCl, also abbreviated as “1/4 NS.” Some orders for IV fluids may therefore be written as D5 ½ NS, D5 ¼ NS. Note: IV fluids can contain saline only (see Figure 21-4) or saline mixed with dextrose, which would be indicated with the percentage of dextrose (e.g., D5 0.9% sodium chloride). Figures 21-3 and 21-5 show saline solution (NaCl) mixed with dextrose. The amount of each ingredient in an IV fluid can be calculated; however, it is not necessary because the label on the IV solution indicates the amount of each ingredient. Calculation of the percentage of solutions was presented in Chapter 5, which deals with percentages.
Intravenous Solutions and Equipment
IV FLUIDS
Calculating Percentage of Solute in IV Fluids


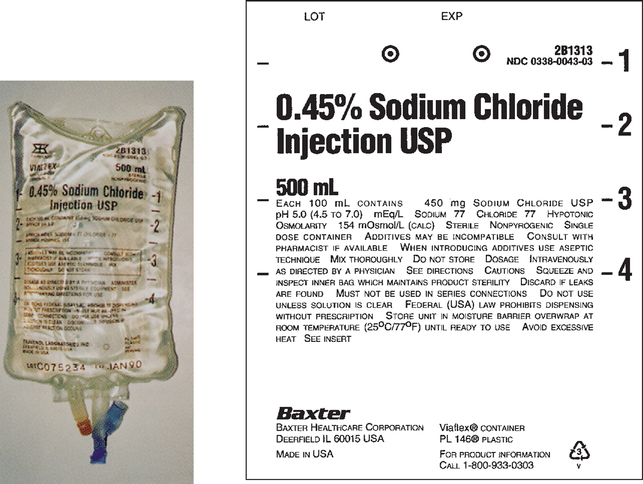
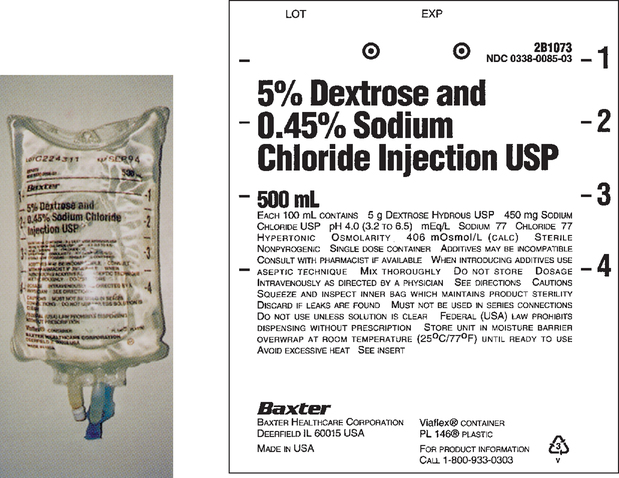
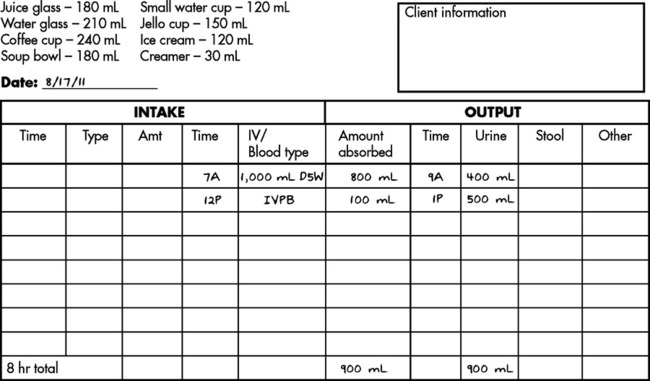
 Solution Using Ratio and Proportion
Solution Using Ratio and Proportion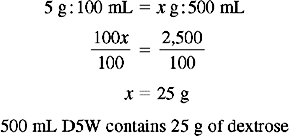
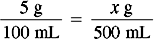
 Solution Using Dimensional Analysis
Solution Using Dimensional Analysis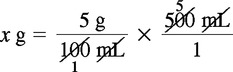

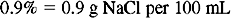
 Solution Using Ratio and Proportion
Solution Using Ratio and Proportion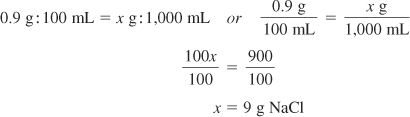
 Solution Using Dimensional Analysis
Solution Using Dimensional Analysis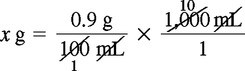
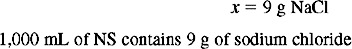
 Solution Using Ratio and Proportion
Solution Using Ratio and Proportion