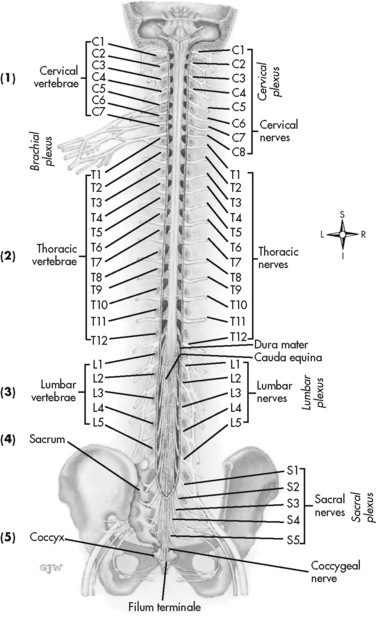
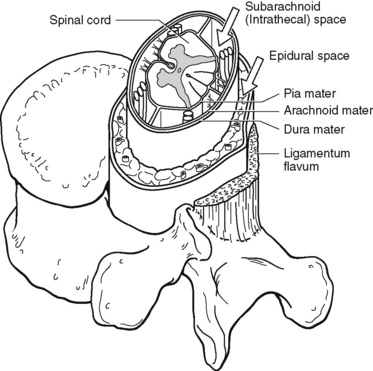
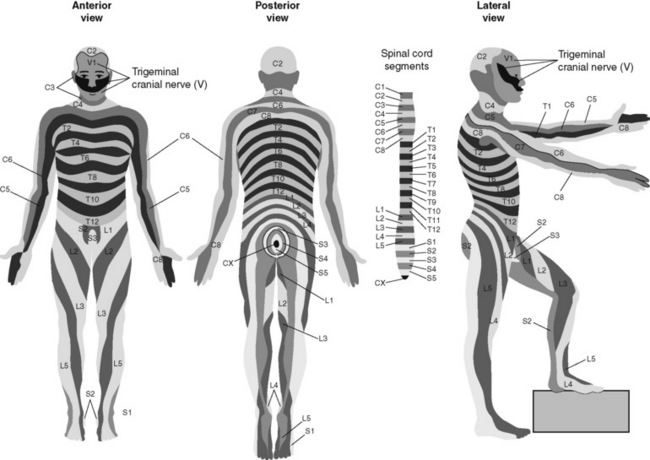
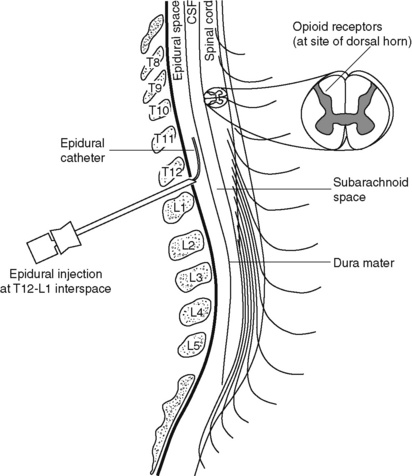
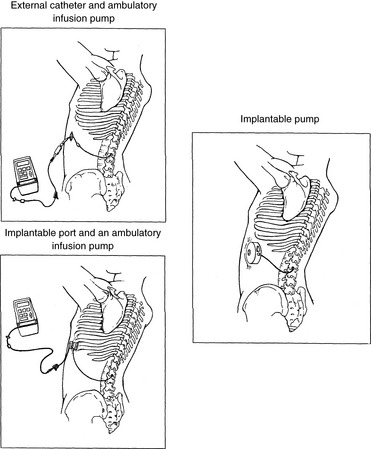
Chapter 15 Delivery of Intraspinal Analgesics Percutaneous Intraspinal Catheterization Intraspinal Analgesia for Persistent Cancer and Noncancer Pain Stability and Compatibility of Agents for Analgesic Infusion Therapy Methods for Administering Intraspinal Analgesia Drug Bioavailability by the Intraspinal Routes Selected Analgesics Administered by the Intraspinal Routes Complications Associated with the Intraspinal Routes of Administration Dural Puncture and Postdural Puncture Headache Direct Needle or Catheter Trauma Injection or Infusion of Neurotoxic Agents Tapering and Cessation of Epidural Analgesia THE term intraspinal refers to the spaces or potential spaces surrounding the spinal cord or the nerve roots that constitute the cauda equina. Most often, the term is used when referring to the epidural and intrathecal spaces, each of which offers a route of administration for medications. The word neuraxial also is used to describe the group of spaces into which analgesic drugs can be administered. The word spinal is used interchangeably with the word intrathecal when referring to route of administration. It may also be used when referring generally to all of the routes of administration near or within the spinal meninges (Swarm, Karanikolas, Cousins, 2004). Intrathecal is often used synonymously with subarachnoid, but anatomically the intrathecal space includes the subdural space (Swarm, Karanikolas, Cousins, 2004) (see the following paragraphs on spinal anatomy). Table 15-1 shows some of the persistent misconceptions related to epidural analgesia. Box 15-1 presents patient selection guidelines and considerations for intraspinal analgesia. Table 15-1 Misconceptions: Epidural Analgesia IM, Intramuscular; IV, intravenous; PCA, patient-controlled analgesia. From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 405, St. Louis, Mosby. Data from American Society of Anesthesiologists Task Force on Neuraxial Opioids. (2009). Practice guidelines for the prevention, detection, and management of respiratory depression associated with neuraxial opioid administration. Anesthesiology, 110(2), 218-230; Brown, D. L. (2005). Spinal, epidural, and caudal anesthesia. In R. D. Miller (Ed.), Miller’s anesthesia, vol 2, ed 6, Philadelphia, Elsevier; Cashman, J. N., & Dolin, S. J. (2004). Respiratory and haemodynamic effects of acute postoperative pain management: Evidence from published data. Br J Anaesth, 93(2), 212-223; Cousins M. J., & Veering, B. T. (1998). Epidural neural blockade. In M. J. Cousins, & P. O. Bridenbaugh (Eds.), Neural blockade in clinical anesthesia and management of pain, Philadelphia, Lippincott-Raven; Dabu-Bondoc, S., Franco, S. A., & Sinatra, R. S. (2009). Neuraxial analgesia with hydromorphone, morphine, and fentanyl: Dosing and safety guidelines. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press; Flisberg, P., Rudin, A., Linner, R., et al. (2003). Pain relief and safety after major surgery. A prospective study of epidural and intravenous analgesia in 2696 patients. Acta Anaesth Scand, 47(4), 457-465; Grape, S., & Schug, S. A. (2008). Epidural and spinal analgesia. In P. E. Macintyre, S. M. Walker, & D. J. Rowbotham (Eds.), Clinical pain management. Acute pain, ed 2, London, Hodder Arnold; Maalouf, D. B., & Liu, S. S. (2009). Clinical application of epidural analgesia. In R. S. Sinatra, O. A. de Leon-Casasola, B. Ginsberg, et al. (Eds.), Acute pain management, Cambridge, NY, Cambridge University Press; McCartney, C. J. L., & Niazi, A. (2006). Use of opioid analgesics in the perioperative period. In G. Shorten, D. B. Carr, D. Harmon, et al., (Eds.), Postoperative pain management: An evidence-based guide to practice, Philadelphia, Saunders; Royse, C. F., Soeding, P. F., & Royse, A. G. (2007). High thoracic epidural analgesia for cardiac surgery: An audit of 874 cases. Anaesth Intensive Care, 35(3), 374-377; Vascello, L., & McQuillan, R. J. (2006). Opioid analgesics and routes of administration. In O.A. de Leon-Casasola (Ed.), Cancer pain. Pharmacological, interventional and palliative care approaches, Philadelphia, Saunders. Pasero C, McCaffery M. May be duplicated for use in clinical practice. The human spinal column consists of 33 individual vertebra referred to by their location: (1) 7 cervical, (2) 12 thoracic, (3) 5 lumbar, (4) 5 caudal or sacral (fused into one bone, the sacrum), and (5) 4 coccygeal (fused into one bone, the coccyx) (Figure 15-1).Vertebrae consist of an anterior body, the laminae that protect the lateral spinal cord, and spinous processes that project outwardly and posteriorly from the laminae. The vertebrae become larger as they descend in the vertebral column. The bones of the laminae are bound together by a number of ligaments (e.g., the dense ligamentum flavum) (Figure 15-2). Moving from outside to inside the spine, the epidural space is first encountered. This is a potential space filled with vasculature, fat, and a network of nerve extensions. No fluid is in the epidural space; a true space is created when volume or air is injected into it (see Figure 15-2). The epidural space is outside of the dura, which is composed of the dura mater and the arachnoid membranes. The subarachnoid space (also called the intrathecal space in the caudal part of the spine) lies deep to the subarachnoid membrane, between this membrane and the spinal cord and cauda equina. The subarachnoid space is filled with clear, colorless cerebrospinal fluid (CSF) that continually circulates and bathes the spinal cord and nerve roots. At each vertebral body level, nerve roots exit from the spinal cord bilaterally. A specific area of skin and subcutaneous tissue, known as a dermatome, is innervated by a single spinal nerve (Figure 15-3). The assessment of sensation in a dermatome is used to determine the integrity of the nerve root and subsequent pathway of innervation. Assessment of sensation in dermatomes is performed by anesthesia providers and others to determine the level of spinal anesthesia for surgical procedures and postoperative analgesia when epidural local anesthetics are used. Delivery of analgesics by the intraspinal routes can be accomplished by inserting a needle into the subarachnoid space (for intrathecal analgesia) or the epidural space and injecting the analgesic, or threading a catheter through the needle and taping it in place temporarily for bolus dosing or continuous administration (Figures 15-4 to 15-6). Temporary catheters are used primarily for short-term acute pain management and are usually removed after 2 to 4 days. Intrathecal catheters for acute pain management are used more often for providing anesthesia and/or a single analgesic bolus dose. For severe persistent cancer and noncancer pain, a catheter can be inserted then tunneled subcutaneously for intrathecal or epidural intermittent bolusing or continuous infusion or for patient-controlled epidural analgesia (PCEA) by an external ambulatory pump. The tunneling is done to decrease the incidence of infection and accidental displacement (Figure 15-7). These temporary tunneled catheters can be used for weeks to months to deliver analgesics. Temporary externalized intrathecal catheters are used less often than temporary epidural catheters primarily because of concerns about infection, although some clinicians report that such concerns may be unfounded (Vascello, McQuillan, 2006). Although temporary tunneled epidural catheters continue to be useful for the management of intractable pain in some patients near end of life, totally implanted intrathecal infusion systems are preferred for long-term treatment of persistent pain (Deer, Krames, Hassenbusch, et al., 2007; Rathmell, Lake, Ramundo, 2006) (see Figure 15-7). Implanted catheters are less likely to dislodge and are associated with a lower infection rate than percutaneous catheters (Rathmell, Lake, Ramundo, 2006; Swarm, Karanikolas, Cousins, 2004) (see more on long-term intraspinal therapy later in the chapter). The level of nociceptive input (e.g., surgical site, site of injury, tumor location), the characteristics of the opioid being administered, and the goals of care (e.g., reduced stress response) are most important in determining the vertebral level at which the catheter is placed (Maalouf, Liu, 2009). For example, long-term catheters for treatment of cancer pain associated with spinal lesions can be placed in a location that avoids the tumor while providing necessary analgesia (DuPen, DuPen, 1998). Temporary epidural catheters for acute pain management usually are placed at the lumbar or thoracic vertebral level depending on surgical site (see the section on dermatomal spread and catheter placement later in the chapter). For example, the high thoracic level is preferred by several clinicians for coronary artery bypass surgery because placement at this level improves coronary perfusion, decreases heart rate and endogenous stress response, and reduces the risk for myocardial ischemia (Kessler, Neidhart, Bremerich, et al., 2002; Paiste, Bjerke, Williams, et al., 2001; Royse, Royse, Soeding, et al., 2000). During intraspinal needle placement, most anesthesia providers are able to recognize when the point of the needle penetrates the dense ligamentum flavum (see Figure 15-2). In addition, entry into the epidural space exerts a negative pressure, which is registered by a loss of resistance in the syringe attached to the needle. Some anesthesia providers use the “hanging-drop” method whereby a drop of fluid at the needle hub is “sucked in” as soon as the needle tip passes the ligamentum flavum (Neruda, 2008); however, this method carries the risk of a small plug in the needle tip creating low or no negative pressure and is discouraged by some practitioners (Cousins, Veering, 1998). Once the ligamentum flavum is penetrated, the needle is not advanced if the epidural space is the desired location. If advanced further, the needle will penetrate the dura and enter the subarachnoid space. When in the subarachnoid space, free-flowing CSF can be aspirated. If a blood vessel is entered during placement, blood often can be aspirated. An early consensus guideline on long-term intrathecal analgesic drug delivery recommended morphine as the mainstay analgesic for long-term intrathecal pain management based on its long history of use, the panel’s extensive clinical experience with the drug, and responses to an online survey of physicians providing long-term intrathecal analgesia (Bennett, Burchiel, Buschser, et al., 2000). The survey revealed a usual starting morphine dose of 0.2 mg/day to 20 mg/day and an average maximum long-term infusion dose of 21.1 mg/day. Updated reviews of the literature and development of algorithms and dosing guidelines for the therapy were published in 2004 (Hassenbusch, Portenoy, Cousins, et al., 2004) and again in 2007 (Deer, Krames, Hassenbusch, et al., 2007). The 2007 recommendations list morphine, hydromorphone, and ziconotide as first-line options. Second-line choices included fentanyl alone, and combinations of morphine/hydromorphone plus ziconotide, or morphine/hydromorphone plus bupivacaine/clonidine. (See Section V for a detailed discussion of ziconotide and other agents administered for long-term intraspinal analgesia.) Table 15-2 provides concentrations and dosing recommendations from the most recent consensus guideline (Deer, Krames, Hassenbusch, et al., 2007). Table 15-2 From Pasero, C., & McCaffery, M. Pain assessment and pharmacologic management, p. 411, St. Louis, Mosby. Data from Deer, T., Krames, E. S., Hassenbusch, S. J., et al. (2007). Polyanalgesic consensus conference: Recommendations for the management of pain by intrathecal (intraspinal) drug delivery; report of an interdisciplinary expert panel. Neuromodulation, 10(4), 300-328. Pasero C, McCaffery M. May be duplicated for use in clinical practice. • Baclofen (Alvarez, de Mazancourt, Chartier-Kastler, et al., 2004; Goodwin, Kim, Zuniga, 2001) • Bupivacaine (Allen, Stiles, Wang, 1993; Classen, Wimbish, Kupiec, 2004; Hildebrand, Elsberry, Deer, 2001b; Nitescu, Hultman, Appelgren, et al., 1992; Rudich, Peng, Dunn, et al., 2004; Tu, Stiles, Allen, 1990; Wulf, Gleim, Mignat, 1994) • Buprenorphine (Nitescu, Hultman, Appelgren, et al., 1992) • Clonidine (Alvarez, de Mazancourt, Chartier-Kastler, et al., 2004; Classen, Wimbish, Kupiec, 2004; Goodwin, Kim, Zuniga, 2001; Hildebrand, Elsberry, Anderson, 2001b; Vranken, van Kan, van der Vegt, 2006; Wulf, Gleim, Mignat, 1994) • Dexamethasone with ketamine (Watson, Lin, Morton, et al., 2005) • Fentanyl (Allen, Stiles, Tu, 1990; Allen, Stiles, Wang, 1993; Chapalain-Pargade, Laville, Paci, et al., 2006; Nitescu, Hultman, Appelgren, et al., 1992; Tu, Stiles, Allen, 1990) • Hydromorphone (Hildebrand, Elsberry, Anderson, 2001b; Rudich, Peng, Dunn, et al., 2004; Walker, Law, DeAngelis, 2001) • Ketamine (Schmid, Koren, Klein, et al., 2002; Walker, Law, DeAngelis, 2001; Watson, Lin, Morton, et al., 2005) • Meperidine (Nitescu, Hultman, Appelgren, et al., 1992; Vranken, van Kan, van der Vegt, 2006) • Morphine (Classen, Wimbish, Kupiec, 2004; Hildebrand, Elsberry, Hassenbusch, 2003; Nitescu, Hultman, Appelgren, et al., 1992; Schmid, Koren, Klein, et al., 2002; Trissel, Pham, 2002; Trissel, Xu, Pham, 2002; Vermiere, Remon, 1999; Wulf, Gleim, Mignat, 1994) • Ropivacaine (Sanchez del Aguila, Jones, Vohra, 2003) • Sufentanil (Boitquin, Hecq, Evrard, et al., 2004; Chapalain-Pargade, Laville, Paci, et al., 2006) • Tramadol with halodroperidol (Negro, Martin, Azuara, et al., 2005) The major drawback of the intermittent epidural bolus method is that a steady analgesic level is difficult to maintain, especially when bolus doses are administered PRN. Relatively large doses of the opioid are given, and a “peak and trough” effect occurs. Patients experience adverse effects at the peak (highest analgesic concentration level) and pain at the trough (lowest analgesic concentration level). Rather than a PRN approach to epidural dosing, it may be preferable to consider smaller scheduled around-the-clock (ATC) doses. A dosing frequency of less than every 6 hours is not recommended (DuPen, DuPen, 1998) (see Box 15-2 for guidelines for administering intermittent boluses through a temporary epidural catheter). The principle of providing continuous pain control with intraspinal analgesia can be accomplished by using an external (for acute pain and for persistent pain) or implanted (for persistent pain) infusion pump to deliver a continuous infusion (also called basal rate) of an analgesic solution. Supplemental bolus doses are prescribed for breakthrough pain and can be administered using the clinician-administered bolus mode available on most external infusion pumps or as outlined in Box 15-2. When implanted ports are used to deliver continuous infusion and/or intermittent boluses, meticulous aseptic precautions should be taken to protect the port from bacterial contamination (DuPen, DuPen, 1998; Holmfred, Vikerfors, Berggren, et al., 2006). When PCEA is administered, a basal rate usually provides most of the patient’s analgesic requirement and the PCEA bolus doses are used to manage breakthrough pain. If a basal rate is not provided, it is especially important to remind patients to “stay on top of the pain,” to maintain a steady neuraxial analgesic level and self-administer bolus doses before pain is severe and out of control. Research has shown that this type of patient teaching is critical to successful therapy (Cywinski, Parker, Xu, et al., 2004). PCEA is safe and effective in older adults (Ishiyama, Iijima, Sugawara, et al., 2007; Mann, Pouzeratte, Boccara, et al., 2000), but the need for proper patient selection and frequent follow up to ensure appropriate PCEA use are emphasized (Silvasti, Pitkanen, 2001). See an example of a patient information brochure about PCEA on pp. 542-543 at the end of Section IV; see Chapter 12 for discussion of PCA principles and safeguards, such as patient-only use of PCA; and see Chapter 17 for discussion of PCA pump features and Table 17-2 on p. 469 for interventions for patients receiving PCEA. More direct delivery of opioids to the site of analgesic action explains why the dose of an opioid by the intraspinal routes is smaller than that required by the parenteral route to produce equal analgesia (i.e., the closer the opioid is delivered to the opioid receptors, the lower the required analgesic dose). For example, research has shown that epidural morphine provides superior analgesia at a lower dose compared with IV or IM morphine; the relative potency of epidural morphine compared with morphine by self-titrated IV PCA was 10:1 following orthopedic surgery (Maalouf, Liu, 2009). When converting opioid-tolerant patients from one route to another, the required dose of morphine is approximately three times less by the epidural route than by the IV route (ratio may vary for other opioids), and the dose required by the intrathecal route is approximately 10 times less than required by the epidural route to produce equal analgesia (DuPen, DuPen, 1998) (see Chapter 18 for switching to different routes of administration).
Intraspinal Analgesia (Epidural and Intrathecal)
Misconception
Correction
Compared with opioid administration via IM injection and IV PCA, the incidence of respiratory depression is higher when opioids are administered by the epidural route.
The incidence of respiratory depression associated with the various pain control methods is not firmly established because of a lack of consensus on definitions and well-controlled research, but the incidence of respiratory depression with epidural analgesia is less than that of IM opioid injections and probably more consistent with that of IV PCA. A systematic review of the literature concluded that the mean reported incidence of opioid-induced respiratory depression varied between 0.8% and 37.0% for IM injection; 1.2% and 11.5% for IV PCA; and 1.1% and 15.0% for epidural analgesia (Cashman, Dolin, 2004). A study of the use of PCEA morphine with basal rate or IV PCA morphine with basal rate in 2696 patients after major surgery reported a higher incidence of respiratory depression with IV PCA (1.2%) than epidural analgesia (0.04%) (Flisberg, Rudin, Linner, et al., 2003). Clinically significant opioid-induced respiratory depression can be avoided in opioid-naïve patients by slow titration, careful nurse monitoring of sedation levels and respiratory status, and decreases in opioid dose when increased sedation is detected (see Chapter 19).
Patients receiving epidural analgesia must be cared for in intensive care settings where their respiratory status can be mechanically monitored.
Patients receiving epidural analgesia have been cared for safely outside of the intensive care setting for many years. Though mechanical monitoring is warranted in patients at high risk for respiratory complications (e.g., those with obstructive sleep apnea, chronic pulmonary disease), nurse assessment of sedation level and respiratory status is reliable and the most common method for monitoring most patients receiving epidural analgesia (see Chapter 19).
Epidural local anesthetics cause excessive and disabling sensory and motor blockade.
Local anesthetics are administered in low (subanesthetic) doses (e.g., 0.05% to 0.125% bupivacaine; 0.1% to 0.2% ropivacaine) for epidural analgesia. Higher doses are required to produce significant motor and sensory blockade (0.5% to 0.75% bupivacaine; 0.75 to 1.0% ropivacaine). Patients receiving epidural analgesia are able to ambulate and perform all the routine recovery activities expected of them to the extent their medical or surgical condition allows. The occasional occurrence of minor temporary numbness of lower extremities is resolved easily by decreasing the dose or removing the local anesthetic from the epidural analgesic solution.
Thoracic epidural catheter placement is technically more difficult and causes more damage than lumbar catheter placement.
The technique for placing a thoracic epidural catheter is quickly mastered by anesthesia providers. A review of 874 cases of high thoracic epidural analgesia provided over a 7-year period revealed no related neurologic complications (Royse, Soeding, Royse, 2007).
Spinal Anatomy
Delivery of Intraspinal Analgesics
Percutaneous Intraspinal Catheterization
Intraspinal Analgesia for Persistent Cancer and Noncancer Pain
Drug
Maximum Concentration
Maximum Dose/Day
Morphine
20 mg/mL
15 mg
Hydromorphone
10 mg/mL
4 mg
Fentanyl
2 mg/mL
No known upper limit
Sufentanil
50 mcg/mL (not available for compounding)
No known upper limit
Bupivacaine
40 mg/mL
30 mg
Clonidine
2 mg/mL
1.0 mg
Ziconotide
100 mcg/mL
19.2 mcg
Stability and Compatibility of Agents for Analgesic Infusion Therapy
Methods for Administering Intraspinal Analgesia
Clinician-Administered Bolus Method
Continuous Infusion
Patient-Controlled Epidural Analgesia (PCEA)
Drug Bioavailability by the Intraspinal Routes
![]()
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


Basicmedical Key
Fastest Basicmedical Insight Engine