Chapter 21 Cataract removal by itself and by whatever technique, be it intracapsular, extracapsular, or phacoemulsification, carries with it its own set of complications, which spare no cataract surgeon—regardless of ability or experience. Intraocular lens (IOL) implantation, whether phakic or aphakic, likewise carries with it its own unique set of complications. These complications can occur before, during, or long after implantation; they can result in damage to the eye, the IOL, or both. Surgeons and manufacturers alike, in an effort to minimize the incidence and severity of IOL-related complications, have been studying implantation techniques and IOL behavior since the late 1970s. The method of insertion of an IOL into the eye, and the IOL design, material, and location are all associated with potential complications. The effect of the location of an IOL inside the eye has been under continuous scrutiny. IOLs have been fixated in the anterior-chamber angle, the anterior iris stroma, the pupil, the posterior-chamber (ciliary sulcus), the lens capsule, and the pars plana. IOL materials have proliferated since the first polymethylmethacrylate (PMMA) IOL was implanted by Harold Ridley1 in 1949. Brought on by the success of small-incision phacoemulsification in the 1970s,2 rigid PMMA lenses have been increasingly supplanted by foldable lenses of silicone, poly-HEMA, acrylic, and biologic collagen.3 Each material behaves differently from the others during and after implantation. The introduction of any physical object through an incision into the eye can result in damage to the intraocular structures (Table 21–1). If an IOL is forced through an incision that is too small, the incision might enlarge by tearing, with the possible result of an unstable wound, requiring suturing to prevent leakage. Wound leakage is thought to be one cause of postoperative infectious endophthalmitis. In an attempt to pass the insertion device through the tight incision, excessive posterior angulation may cause inadvertent iridodialysis, with associated hyphema. Similarly, an insertion forceps, or cartridge angled too anteriorally might cause stripping of Descemet’s membrane. The improper wound and chamber dynamics thus created could result in iris prolapse, chamber collapse, corneal endothelial damage, and subsequent postoperative corneal edema. Improper attention during implantation may traumatize the capsule, resulting in zonulodialysis, tearing of the anterior capsule, tearing of the posterior capsule, and rupture of the anterior hyaloid face, increasing the incidence of postoperative cystoid macular edema (CME) and retinal detachment. With proper attention paid to wound size and construction, chamber maintenance, and careful intraocular manipulation, the incidence of traumatic IOL implantation can be greatly minimized. Just as the ocular tissues may be damaged during IOL implantation, so may the IOL itself (Table 21–2). Damage to an IOL ranges from minor implant forceps scratches on the optic surfaces of a PMMA IOL and scuffing of the surface of acrylic or silicone, to major optic fracture or transection in silicone IOLs. An IOL haptic may also be deformed or fractured.
INTRAOCULAR LENS
IMPLANTATION
OPERATIVE OCULAR COMPLICATIONS
OPERATIVE IOL COMPLICATIONS
Unstable wound/leakage Stripped Descemet’s membrane Corneal endothelial trauma Iris prolapse Iridodialysis Hyphema Zonulodialysis Torn anterior capsule Torn posterior capsule Rupture anterior hyaloid |
Certain PMMA loop haptics, particularly one-piece designs but also the plate haptics of silicone IOLs, are prone to breaking off during insertion. The fracture of a PMMA loop haptic occurs with excessive flexion of a loop, often during insertion through a tight incision. A lens sustaining such damage becomes unstable. It is mandatory that it be replaced.
Three-piece foldable IOLs may sustain damage to the haptics during implantation. When using forceps the leading or trailing haptic may be caught by the folder and crimped. A bend in the haptic can be gently straightened and the IOL implanted. If it fractures or is broken during the straightening process, it must be removed and replaced. Rarely, when injecting three-piece lenses, if the lens is not positioned properly in the cartridge, the leading haptic may be torn off during implantation. This IOL also must be removed and replaced. The trailing haptic may be crimped by improper plunger positioning. This can usually be straightened, but if severe, or broken, the IOL must be removed and replaced.
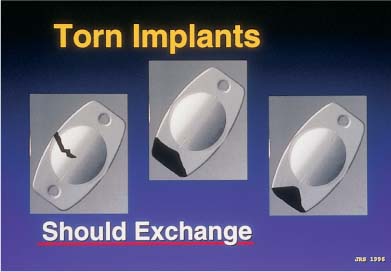
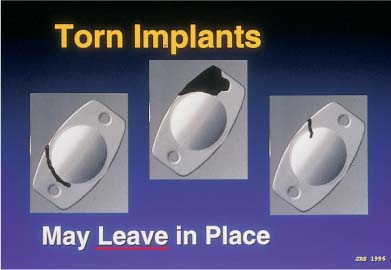
Silicone three-piece IOLs that are left in the barrel of the injector for too long will often stick to the plastic cartridge and tear during insertion. It is therefore important not to load the IOL into the cartridge more than 5 minutes prior to insertion, and not to start the IOL down the barrel until the actual time of intended insertion (Table 21–3). Plate haptic silicone 6.0-mm optic lenses were originally designed to go through cartridges large enough for 4.0-mm scleral incisions. However, they are now going through cartridges tapered to fit through sub–3.0-mm clear-corneal incisions. Subsequent compression and occasional piston override can result in either haptic or optic fracture. If the fracture has completely transected the trailing plate haptic, destabilizing the optic centration, the IOL must be removed and replaced (Fig. 21–1). However, if the fracture involved avulsion of only the corner of a plate or is only partially through a haptic, the lens may be left in situ, as the centration of the IOL will not be affected (Fig. 21–2). A partial fracture of an optic that does not involve the central 3 to 4 mm of the “optical zone” may also be left in place. In these cases, rotation of the peripheral optic fracture to the 12 o’clock position will allow the upper eyelid to cover the peripheral optic aberration. In addition, capsulorrhexis contraction and fibrosis may cover the area (Fig. 21–3), as well as the pupil itself.
Haptic deformation Haptic fracture Haptic avulsion Optic excoriation Optic deformation Optic fracture Asymmetric bag/sulcus implantation Reversed-optic implantation* Off-axis implantation** Posterior IOL dislocation |
*Angled haptics, toric, and multifocal IOLs.
Problem | Cause | Management |
Torn leading or trailing haptic | Improper placement in cartridge Inadequate viscoelastic Aggressive injector speed | Careful observance of placement guide Adequate viscoelastic Inject more slowly |
Crimped trailing haptic | Plunger damage | Watch plunger as it contacts posterior IOL, avoid contact with haptic |
Torn IOL | Improper placement in cartridge Inadequate viscoelastic Aggressive injector speed Too long dwell time | Careful observance of placement guide Adequate viscoelastic Inject more slowly Do not load IOL into cartridge more than 5 minutes before intended insertion |
FIGURE 21–1 Silicone plate-haptic intraocular lens (IOL) damage on injection, causing optic fracture or unstable haptic avulsion. (Courtesy of John R. Shepherd.)
PHAKIC IOL
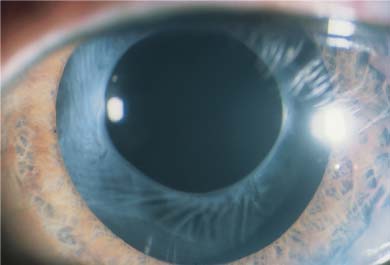
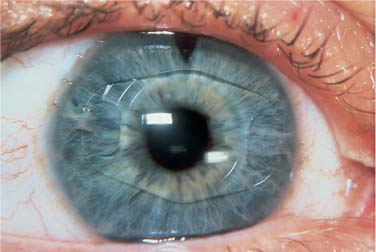
Errors of implantation may also occur without damage to the IOL. Although anterior chamber (AC) IOL implantation is now rarely performed during cataract surgery, it is currently under active investigation for the phakic correction of ametropia. As the pupil is often intentionally miotic for anterior chamber implantation, damage to the iris may occur. Iridodialysis with hyphema can occur both on the proximal side, due to dragging of the subincisional iris while moving the leading haptic toward the pupil, and on the distal side, when attempting to force the trailing haptic of an oversized IOL into the proximal angle. Tucking of the distal iris causes an acute ovaling of the pupil (Fig. 21–4). Excessive manipulation of an AC IOL may result in chamber collapse with IOL contact with the corneal endothelium.
FIGURE 21–2 Silicone plate-haptic IOL damage on injection, not affecting IOL stability or optical performance. (Courtesy of John R. Shepherd.)
FIGURE 21–3 Anterior capsular fibrosis over plate-haptic silicone IOL.
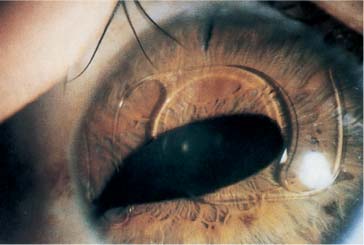
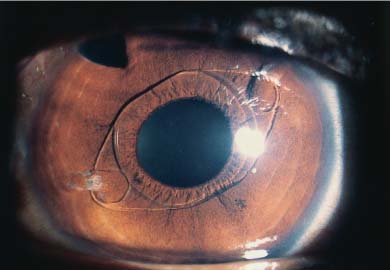
Phakic and aphakic iris-supported IOL implantation is also currently in its second generation. The Worst-Fechner “lobster-claw” IOL4 (Artisan, Ophtec) (Fig. 21–5) and the version designed by Daljit Singh (Amritsar, India) (Fig. 21–6) are enclaved onto the anterior iris stroma, unlike their first-generation predecessors—the Binkhorst “iris-clip” IOL, the Worst “two-loop medallion” IOL, the Copeland “Maltese cross” IOL, the Fyodorov “Sputnik” IOL, and others—which were pupil-supported. The enclavation process of these newer version iris-supported IOLs can result, in the event of phakic implantation, in pressure damage to the lens in back with subsequent development of cataract, or to endothelial damage in both phakic and aphakic implantation if care is not taken to suture or secure the wound before enclavation in order to prevent viscoelastic escape and chamber collapse (assuming that proper stabilization of the IOL was maintained).
FIGURE 21–4 Progressive postoperative pupillary ovalization with a polymethylmethacrylate (PMMA) anterior chamber (AC) IOL. (Courtesy of J. Alio.)
FIGURE 21–5 Worst-Fechner Artisan (Ophtec) PMMA iris stromal AC IOL. (Courtesy of J. Worst.)
POSTERIOR CHAMBER IOL
Implantation of the posterior chamber presumes the presence of an intact or mostly intact zonulocapsular apparatus. The zonule may be complete or incomplete. The capsule may be intact or partially collapsed from sectoral zonulodialysis or torn anteriorly or posteriorly. Just as an AC IOL can tuck the peripheral anterior iris stroma, so can a posterior chamber (PC) IOL tuck the peripheral posterior iris pigmented layer. The haptic, in this case, may push the iris forward at that location, causing sectoral angle closure and peripheral anterior synechia formation. Chronic chafing of the pigmented layer can result in a transillumination defect and pigment dispersion syndrome. Vigorous implantation of rigid haptics into the ciliary sulcus, particularly those of long overall diameter one-piece PMMA PC IOLs, can result in traumatic zonulodialysis and inadvertent posterior loop location on the pars plana.5,6 Implantation in the posterior chamber in the absence of capsular support requires suturing of loops to the sclera.7,8 Avoiding damage to the ciliary sulcus structures by the blind passage of a needle behind the iris can be facilitated by passing a hollow needle ab externo to guide the intraocular suture needle out through the proper anatomic location9–11 (see Chapter 22).
FIGURE 21–6 PMMA iris stromal AC IOL. (Courtesy of D. Singh.)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree