Objectives
- Understand the role played by the mucosal immune system in protecting the host from infections acquired via the oral route while failing to respond to innocuous antigens
- Define the mechanisms and relative roles of innate versus adaptive immunity
- Understand the specialized features of the mucosal immune system compared with immunity expressed in the periphery
- Define the mechanisms and relative roles of innate versus adaptive immunity
- Identify the cell populations that contribute to immunity in the gut and their locations
- Describe mechanisms that result in cellular homing to the gut and other mucosal sites
- Describe the characteristics of IgA that make it especially suited to function in the gut
- Describe mechanisms that result in cellular homing to the gut and other mucosal sites
- Describe the immune responses that occur to antigens encountered in the gut
- Understand the concept of oral tolerance
- Understand the origins, make-up and physiological importance of microbial populations that exist in the normal intestine
- Define the consequences of abnormal immune responses in the intestine
Basic Principles of Mucosal Immunology
 As we have learned in previous chapters, the surface of the gastrointestinal tract represents a vast frontier that can potentially serve as a portal of entry into the body. Moreover, by the very nature of the physiological function of the gut, its lumen is frequently filled with a complex mixture of nutrients that constitute an attractive “culture medium” for a variety of microbes. Indeed, the intestine is challenged to distinguish between potentially harmful microorganisms, against which it must defend itself, versus the innocuous antigens that occur in food. The intestine also has a special need for immune surveillance against malignancy. Thus, the rapid rate of proliferation of intestinal epithelial cells, coupled with exposure of these cells to potential toxins in the intestinal lumen, renders the epithelium uniquely sensitive to cell transformation. It is likely that the immune system is important in detecting many transformed cells before they have the opportunity to develop into a tumor, although clearly this line of defense is not perfect. Finally, over millennia, humans and other animals have been exposed to a barrage of intestinal pathogens via contaminated food and water; inadequate sanitation still persists in many underdeveloped areas of the world. This constant exposure has driven the development of a highly specialized branch of the immune system, referred to as the mucosal immune system, which encompasses the mucosa-associated lymphoid tissues, or MALT.
As we have learned in previous chapters, the surface of the gastrointestinal tract represents a vast frontier that can potentially serve as a portal of entry into the body. Moreover, by the very nature of the physiological function of the gut, its lumen is frequently filled with a complex mixture of nutrients that constitute an attractive “culture medium” for a variety of microbes. Indeed, the intestine is challenged to distinguish between potentially harmful microorganisms, against which it must defend itself, versus the innocuous antigens that occur in food. The intestine also has a special need for immune surveillance against malignancy. Thus, the rapid rate of proliferation of intestinal epithelial cells, coupled with exposure of these cells to potential toxins in the intestinal lumen, renders the epithelium uniquely sensitive to cell transformation. It is likely that the immune system is important in detecting many transformed cells before they have the opportunity to develop into a tumor, although clearly this line of defense is not perfect. Finally, over millennia, humans and other animals have been exposed to a barrage of intestinal pathogens via contaminated food and water; inadequate sanitation still persists in many underdeveloped areas of the world. This constant exposure has driven the development of a highly specialized branch of the immune system, referred to as the mucosal immune system, which encompasses the mucosa-associated lymphoid tissues, or MALT.
In fact, the intestine represents the largest immunological compartment of the body, and has also evolved nonimmunologic barriers to invasion by pathogens. The nonimmune barriers include secretion of the acid by the stomach, the potential antimicrobial actions of other components of the digestive juices such as enzymes and bile acids, the mucus layer, which overlies much of the epithelium, that limits microbial attachment to the epithelial surface, specific antibacterial products secreted by specialized epithelial cells or by the salivary glands, and the epithelium itself, which, when intact, represents a physical barrier to the uncontrolled flux of microbes into the body. The immune barriers include both the so-called innate immune system, and adaptive, or acquired immunity. Innate immunity consists of a series of mechanisms to respond to molecular structures that are broadly specific for classes of microbes, but are not expressed by host cells. These structures are referred to as pathogen-associated molecular patterns, and are recognized by pattern-recognition receptors. This event sets in motion a number of rapid responses that are designed to repel invading pathogens. In contrast, adaptive immunity develops more slowly, but is exquisitely specific, potentially more effective, and generates a “memory” in the host that permits an amplified response if the same pathogen is encountered again. Adaptive immunity is mediated by lymphoid cells, notably T and B lymphocytes, and soluble antibodies. Overall, the combined activity of the more primitive, or ancient, innate system, and adaptive immunity, is remarkably effective in protecting us from the potential perils of intestinal infections.
 The intestinal immune system, particularly with respect to the adaptive limb, is also part of a broader immune system that protects other mucosal surfaces in the body, including the airways, eyes, urogenital tracts, and the mammary glands. Lymphocytes specific for antigens encountered via any of these mucosal routes undergo regulated trafficking that allows them to engage in homing back not only to the site where their expansion was stimulated, but also to all of the other mucosal sites listed. This provides a common system of mucosal protection. The concept of the common mucosal immune system is also relevant to immune protection of neonates by maternal antibodies. The majority of antibodies secreted in breast milk are specific for antigens that the mother has encountered via the gut, and provide similar protection to the baby, whose immune system is not mature at birth.
The intestinal immune system, particularly with respect to the adaptive limb, is also part of a broader immune system that protects other mucosal surfaces in the body, including the airways, eyes, urogenital tracts, and the mammary glands. Lymphocytes specific for antigens encountered via any of these mucosal routes undergo regulated trafficking that allows them to engage in homing back not only to the site where their expansion was stimulated, but also to all of the other mucosal sites listed. This provides a common system of mucosal protection. The concept of the common mucosal immune system is also relevant to immune protection of neonates by maternal antibodies. The majority of antibodies secreted in breast milk are specific for antigens that the mother has encountered via the gut, and provide similar protection to the baby, whose immune system is not mature at birth.
 The immune system in the intestine is specialized to subserve the specific functions discussed earlier. First, in conditions of health, the lymphocytes that traffic to mucosal sites encounter antigens in a controlled fashion. This is accomplished by limiting the uptake of significant quantities of particulate antigens only to specific sites within the epithelial monolayer, via specialized epithelial cells known as M cells. M cells overlie organized lymphoid aggregates known as Peyer’s patches. The lymphocytes in these structures are immunologically naive and represent the afferent arm of the mucosal immune system; after they have been stimulated by their cognate antigen, they traffic back to the lamina propria via draining lymph nodes, the thoracic ducts, and the bloodstream, from where they reenter the mucosa. During this migration the lymphocytes mature and differentiate, and then represent an efferent arm of the system, capable of effector functions in the mucosa. Second, unlike the peripheral immune system, where the primary immunoglobulin is IgG, the humoral aspects of mucosal immunity are predominantly served by secretory IgA molecules, which will be discussed in more detail later. Third, the intestinal mucosa can be considered to be constantly in a state of “physiological” inflammation, even in health. Presumably this reflects the constant stimulation the system receives from the intestinal microflora, and renders the intestine armed and ready to respond rapidly at times of threat by pathogens. Finally, the specific lymphocyte subsets present in the intestine can promote a particular type of immune response called oral tolerance, where a local antibody response to a specific antigen can be mounted at mucosal sites in the absence of a response in the periphery.
The immune system in the intestine is specialized to subserve the specific functions discussed earlier. First, in conditions of health, the lymphocytes that traffic to mucosal sites encounter antigens in a controlled fashion. This is accomplished by limiting the uptake of significant quantities of particulate antigens only to specific sites within the epithelial monolayer, via specialized epithelial cells known as M cells. M cells overlie organized lymphoid aggregates known as Peyer’s patches. The lymphocytes in these structures are immunologically naive and represent the afferent arm of the mucosal immune system; after they have been stimulated by their cognate antigen, they traffic back to the lamina propria via draining lymph nodes, the thoracic ducts, and the bloodstream, from where they reenter the mucosa. During this migration the lymphocytes mature and differentiate, and then represent an efferent arm of the system, capable of effector functions in the mucosa. Second, unlike the peripheral immune system, where the primary immunoglobulin is IgG, the humoral aspects of mucosal immunity are predominantly served by secretory IgA molecules, which will be discussed in more detail later. Third, the intestinal mucosa can be considered to be constantly in a state of “physiological” inflammation, even in health. Presumably this reflects the constant stimulation the system receives from the intestinal microflora, and renders the intestine armed and ready to respond rapidly at times of threat by pathogens. Finally, the specific lymphocyte subsets present in the intestine can promote a particular type of immune response called oral tolerance, where a local antibody response to a specific antigen can be mounted at mucosal sites in the absence of a response in the periphery.
Functional Anatomy of the Mucosal Immune System
As discussed earlier, the innate arm of the mucosal immune system is designed to mount rapid responses to pathogens, and does so by expressing pattern-recognition receptors that recognize molecules that are important to broad classes of pathogenic microbes, such as lipopolysaccharides and peptidoglycans. Macrophages represent one important class of effectors of such innate immune responses, but in fact pattern-recognition receptors may be more widely distributed, including on cells not classically considered to be immune effector cells, such as the epithelium. Pattern-recognition receptors include the Toll-like receptors, so-called because of their homology to the Drosophila defense molecule, Toll, and other proteins that may respond to pathogen molecules presented intracellularly, such as Nod 1 and Nod 2. In general, activation of the innate immune response generates chemotactic molecules that stimulate the influx and activation of further inflammatory cells, including additional monocytes that can differentiate into tissue macrophages, as well as, importantly, neutrophils. Collectively, these cells can effect microbial killing by releasing a variety of toxic products, including reactive oxygen species, which have the unfortunate side effect that they may also cause bystander damage to adjacent, uninfected tissues. Activation of the innate immune response may also generate cytokines that facilitate the later, and more specific, adaptive immune response.
Unlike the limited selection of pathogen factors recognized by innate immunity, adaptive immunity involves the exquisitely specific recognition of literally millions of discrete antigenic sequences that are found in microorganisms as well as in abnormal host cells, such as those that have undergone malignant transformation or which are virally infected. Such recognition is mediated by specific receptors expressed on two classes of lymphoid cells, T and B cells. T cells recognize peptides derived from antigenic sequences via a heterodimeric, variable cell surface T-cell receptor that originates from recombination of various distinct gene segments as well as subsequent editing to provide for additional diversity. The peptides are presented bound to major histocompatibility complex (MHC) molecules on antigen-presenting cells, which include dendritic cells and likely intestinal epithelial cells. A positive response also requires costimulation of the T cell via accessory molecules and ligands. The binding of antigen to a specific T-cell receptor then drives the expansion of a clone of cells expressing that receptor; some of these differentiate into effector T cells capable of secreting cytokines that regulate additional immune responses, others remain as memory cells to jump-start an adaptive immune response if the same antigen is encountered again. T cells in the mucosal immune system can be subdivided into those expressing the differentiation marker CD4, and those expressing CD8. The former cell population recognizes antigens, derived from the extracellular environment via endocytosis, that are displayed on the surface of antigen-presenting cells in the context of MHC class II molecules. Such antigens likely include components of pathogenic microorganisms. In general, CD4-positive T cells help to regulate immune responses, and also further differentiate into specific subsets that secrete characteristic products depending on the specific threat to the system, under the influence of specific cytokine messages. CD4-positive cells can further be subdivided into effector and regulatory subsets. The effector cells are so-called “helper” cells (Th) that promote the development of B cells and thereby “help” them to produce antibodies. Regulatory T cells, on the other hand, suppress the activity of other T cells at sites of inflammation or in response to the commensal microbiota. There is evidence that some inflammatory diseases of the intestine may arise, in part, from the failure to develop sufficient numbers of regulatory T cells. CD8 positive T cells, on the other hand, recognize abnormal intracellular proteins in the context of MHC class I molecules. Like CD4 cells, CD8 cells may generate cytokines that regulate the network of immune responses, but CD8 cells may also be directly cytotoxic. Thus, CD8 T cells provide important protection against potentially harmful intracellular events, such as viral infection or malignant transformation. MHC class I molecules are expressed on essentially all cell types in the gut, underscoring the significance of this protective pathway.
Adaptive immunity is also mediated by B cells, which differentiate into plasma cells and begin to secrete antibodies specific for a given antigen under the influence of antigen-specific T cells and the cytokines they produce, especially transforming growth factor-β, interleukin (IL)-4, IL-5, and IL-6. This exemplifies a central principle in intestinal adaptive immunity, which is that an effective immune response typically requires the cooperation of several different cell types as well as soluble mediators. Antibodies produced by B cells may additionally mediate activity of another class of effector cells known as natural killer or NK cells. These cells represent a link between the adaptive and innate branches of the immune response. NK cells can destroy particles (e.g., microbes) that have been opsonized, i.e., coated with antibodies specific for cell surface components. However, NK cells also exert cytotoxic effects that are independent of any aspects of the adaptive response. Thus, they recognize cells that have downregulated MHC class I molecules, which is a strategy commonly employed by virally infected or tumor cells in an attempt to evade the immune attack that would otherwise be mounted by CD8-positive T cells. In either case, however, NK cells lyse their targets via the release of cytotoxic products that include an enzyme, granzyme, and a substance capable of forming pores in target cell membranes, called perforin.
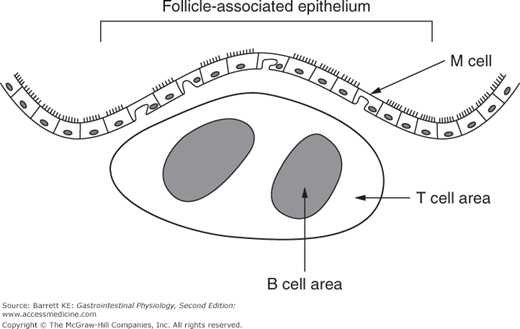
It is also important to appreciate how lymphoid tissues are organized in the intestine. As mentioned earlier, in the small intestine, the afferent arm of the adaptive system (i.e., the arm that responds initially to a threat) occurs in structures known as Peyer’s patches, as shown diagrammatically in Figure 6–1. In the colon, these aggregates of lymphocytes are more loosely organized, but analogous in function. Naive T and B cells from the bloodstream are targeted to migrate into Peyer’s patches because they recognize a specific type of endothelial cell that is found in the blood supply to these lymphoid structures. The other important components of the Peyer’s patch include the M cell, which replaces normal enterocytes as an epithelial covering for the lymphoid follicle, and is important in initial uptake of luminal particulates, and dendritic cells and macrophages, which serve to process and present antigens to the T and B cells. Once stimulated, activated T and B cells migrate out of the lymphoid follicle and, via the pathways discussed earlier, eventually back to the lamina propria.
Figure 6–1.
Structure of a Peyer’s patch in the small intestinal mucosa. The follicle-associated epithelium contains M, or microfold cells, that have a subapical pocket in which antigens can be presented to immune cells. Lymphocytes are aggregated underneath the epithelium with T and B lymphocytes restricted to distinct areas. Peyer’s patchers also contain dendritic cells (not shown), which can present antigens to lymphocytes.
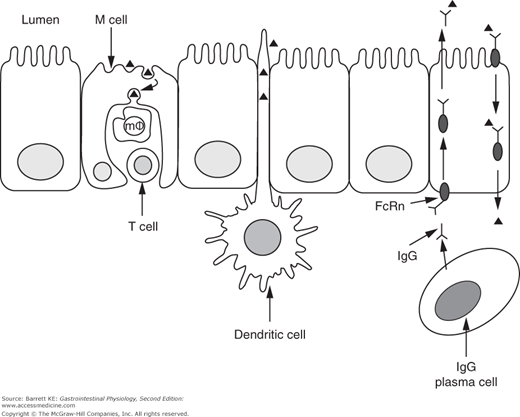
Dendritic cells are also more widely distributed in the intestinal mucosa beyond the Peyer’s patches. They are thought to be important in additional sensing/sampling of luminal antigens, because they can project their dendrites between the tight junctions of adjacent epithelial cells. The antigens thus taken up are presented to lymphocytes in the lamina propria (Figure 6–2).
Figure 6–2.
Pathways for antigen uptake across the intestinal epithelium. Proteins (small triangles) are taken up by M cells or the processes of dendritic cells, or after binding to specific IgG molecules that are subsequently trafficked in a retrograde fashion by the neonatal Fc receptor (FcRn) expressed on intestinal epithelial cells. m , macrophage.
, macrophage.
A final class of organized lymphoid cells in the intestine consists of a subset of lymphocytes that are anchored immediately adjacent to the epithelial layer via specific adhesion molecules. These are called intraepithelial lymphocytes, and appear predominantly to consist of memory T cells capable of responding to only a subset of luminal antigens. They likely function primarily to secrete cytokines involved in the regulation of the epithelium, and may also participate in immune surveillance for emerging malignancies.
Secretory IgA System
Effector B cells in the lamina propria are capable of synthesizing a range of immunoglobulins. Following maturation into a plasma cell, a given B cell retains the ability to synthesize only one specific antibody type. However, the distribution of immunoglobulin subtypes in the mucosal immune system is quite different from that seen in the peripheral immune system, as represented by circulating B cells. Thus, while IgG is the major antibody in the bloodstream, in the intestine, 70–90% of the B cells in the lamina propria secrete IgA, with the remainder largely making IgM, and a few cells making IgE. Very few cells in the lamina propria make IgG in healthy individuals. In fact, given the large numbers of lymphocytes that normally reside in the gut, the daily synthesis of IgA exceeds that of all other immunoglobulins.
There are two subclasses of IgA, coded by separate genes in the immunoglobulin locus. IgA1 is the predominant form of this antibody found in the circulation, and the majority of serum IgA is also in the form of monomers. On the other hand, the IgA plasma cells that localize to the intestinal lamina propria show a greater predominance of IgA2, and essentially all of the IgA is in the form of dimers.
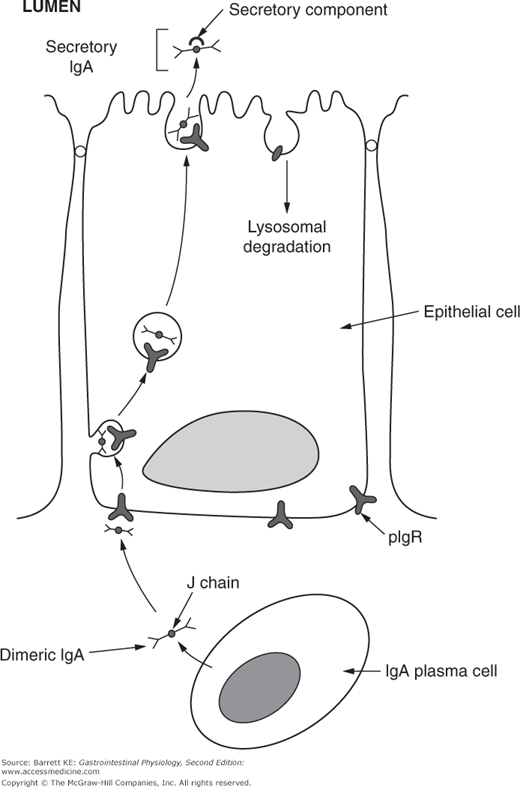
The two IgA molecules in dimeric IgA are bound together by a short polypeptide sequence known as the J (or joining) chain. J chain is also a component of other polymeric immunoglobulins, such as IgM. The other critical component of secretory IgA that is found in the lumen of the intestine derives from intestinal epithelial cells (Figure 6–3). Thus, dimers of IgA plus J chain are taken up at the basolateral surface of the epithelium by binding to a structure known as the polymeric immunoglobulin receptor, or pIgR. The complex of IgA plus pIgR is internalized and translocated across the epithelial cell. At the apical membrane, the IgA dimer is released into the lumen bound to a cleaved portion of pIgR, known as secretory component. Secretory component stabilizes the IgA dimer against proteolytic cleavage by either the digestive juices or bacterial proteases.
Figure 6–3.
Secretion of IgA across the intestinal epithelium. IgA is secreted by plasma cells in the lamina propria as a dimer, with two IgA molecules linked by a J, or joining, chain. J chain is recognized by the polymeric immunoglobulin receptor (pIgR) expressed on the basolateral membrane of epithelial cells, and once bound, the complex is internalized and trafficked across the cytosol to the apical membrane. Apical proteases cleave the extracellular portion of pIgR, which remains associated with the IgA dimer as secretory component. The remnant of the pIgR is internalized and degraded.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree