Objectives
- Understand how the motility functions of the small intestine and colon contribute to the integrated response to a meal
- Describe the muscle layers and their connections to the enteric nervous system that subserve intestinal motility
- Identify the sphincters that control movement of intestinal contents between segments, or out of the body
- Describe the muscle layers and their connections to the enteric nervous system that subserve intestinal motility
- Define the motility patterns that characterize movements of the small and large intestines under fed and fasted conditions and their control mechanisms
- Distinguish between mixing patterns and those that propel contents along the length of the intestine
- Describe reflexes that coordinate the motility functions of the small intestine and colon with the function of the stomach
- Understand the process whereby undigestible residues of the meal are eliminated from the body
- Distinguish between mixing patterns and those that propel contents along the length of the intestine
- Understand the pathophysiology of disease states where intestinal motility is abnormal
Basic Principles of Intestinal Motility
The ability of the walls of the small and large intestines to contract and relax allows for the movement of intestinal contents from one site to another. Specific motility patterns subserve the functions of each intestinal segment. In addition, specialized muscle regions, or sphincters, retard the passage of intestinal contents in a controlled fashion at specific sites.
 As we have learned from previous chapters, the primary role of the small intestine is to digest the various components of the meal and to absorb the resulting nutrients into the bloodstream or lymphatic system. The motility patterns observed in the small intestine are profoundly altered by eating. The duration of such changes depends on the caloric load and the type of nutrients ingested, with lipids having the most durable effect. During the fed state, many of the motility patterns in the small intestine are designed not to propel the intestinal contents aborally, but rather to mix the contents with the various digestive secretions and prolong their exposure to the absorptive epithelium. The muscle layers of the small intestine interact to provide for “two steps forward and one step back,” retaining the intestinal contents long enough to provide for efficient extraction of most or all useful substances. In general, therefore, the motility functions of the small intestine control the rate of nutrient absorption. The speed with which the contents are propelled also varies along the length of the small intestine. Movement is fastest in the duodenum and jejunum, providing for rapid mixing and propulsion of the contents both orally and aborally. Motility then slows in the ileum, providing additional time for the absorption of slowly permeable nutrients, and particularly, lipids. Then, once the meal is digested and absorbed, the small intestine converts to the migrating motor complex (MMC) we also discussed for the stomach, a pattern of relative quiescence punctuated by propulsive motility patterns that expel undigested residues through the small intestine and into the colon.
As we have learned from previous chapters, the primary role of the small intestine is to digest the various components of the meal and to absorb the resulting nutrients into the bloodstream or lymphatic system. The motility patterns observed in the small intestine are profoundly altered by eating. The duration of such changes depends on the caloric load and the type of nutrients ingested, with lipids having the most durable effect. During the fed state, many of the motility patterns in the small intestine are designed not to propel the intestinal contents aborally, but rather to mix the contents with the various digestive secretions and prolong their exposure to the absorptive epithelium. The muscle layers of the small intestine interact to provide for “two steps forward and one step back,” retaining the intestinal contents long enough to provide for efficient extraction of most or all useful substances. In general, therefore, the motility functions of the small intestine control the rate of nutrient absorption. The speed with which the contents are propelled also varies along the length of the small intestine. Movement is fastest in the duodenum and jejunum, providing for rapid mixing and propulsion of the contents both orally and aborally. Motility then slows in the ileum, providing additional time for the absorption of slowly permeable nutrients, and particularly, lipids. Then, once the meal is digested and absorbed, the small intestine converts to the migrating motor complex (MMC) we also discussed for the stomach, a pattern of relative quiescence punctuated by propulsive motility patterns that expel undigested residues through the small intestine and into the colon.
The functions of the colon are quite distinct from those of the small intestine. Thus, while the colon does engage in some limited digestion and salvage of nutrients from undigested residues, with the cooperation of its endogenous flora, the primary functions of the colon are to extract and reclaim water from the intestinal contents, and to process the feces for elimination. As a result, even in the fasted state, the motility functions of particularly the ascending and transverse colon are considerably more biased toward mixing the contents and retaining them for prolonged periods, and the colon does not participate in the MMC. On the other hand, periodically, large propulsive contractions sweep through the colon, transferring its contents to the rectum and ultimately promoting the urge to defecate.
Functional Anatomy
The small intestine, a hollow tube approximately 600 cm in length in a normal adult, is surrounded by two overlapping muscle layers that together make up the muscularis externa. A layer of circular muscle is found closest to the mucosa, overlaid by a longitudinal muscle layer. Taken together, these muscle layers can produce most, if not all, of the stereotypical motility patterns of the small intestine. There is also a thin layer of muscle sandwiched between the mucosa and submucosa, the muscularis mucosa, but the contribution of this muscle layer to the bulk motility properties of the small intestine is unclear. Instead, it may confer specific motility functions on mucosal structures, such as the villi.
The functions of the circular and longitudinal muscle layers are closely integrated. In part, this derives from the fact that they engage in a high level of electrical coupling. Structures known as gap junctions, which permit small second messengers and electrical signals to be communicated between adjacent cells, mean that stimulation of one smooth muscle cell can rapidly be transmitted to its neighbors, without the need for additional neural input. The function of the two muscle layers is also coordinated by the activity of interstitial cells of Cajal. These cells undergo rhythmic cycles of depolarization, related to oscillations in intracellular calcium concentration. As in the stomach, these cells provide the pacemaker function that dictates the basal electrical rhythm, or slow waves, that ultimately control the rate and locations of phasic contractions of the smooth muscle. In the duodenum, the basal electric rhythm occurs at a rate of 12 cycles per minute (cpm), although this slows as one moves distally to 7–8 cpm in the distal ileum. Interstitial cells of Cajal are essential for the peristaltic reflex in the small intestine (and to a lesser extent in the colon) and their numbers may be reduced under conditions associated with slowed transit, such as constipation.
The large intestine also contains both circular and longitudinal muscle layers that regulate its motility, but the anatomic arrangement of these differs somewhat from that seen in the small intestine. In the ascending, transverse, and descending colon, the circular muscle layer is overlaid by three long nonoverlapping bands of longitudinal muscle oriented at 120° to each other, known as the taeniae coli. Electrical coupling between the circular muscle and taeniae coli is less effective than seen in between the corresponding muscle layers in the small intestine, which likely contributes to less effective propulsive motility. The circular muscle layer is also contracted intermittently to divide the colon into functional segments known as haustra. The speed of impulse propagation is faster in the circular muscle of the colon than in the longitudinal layers, allowing for these segmenting ring contractions. Note that the haustral segments are not permanent structures, however, and thus they can be smoothed out to permit propulsion of the colonic contents.
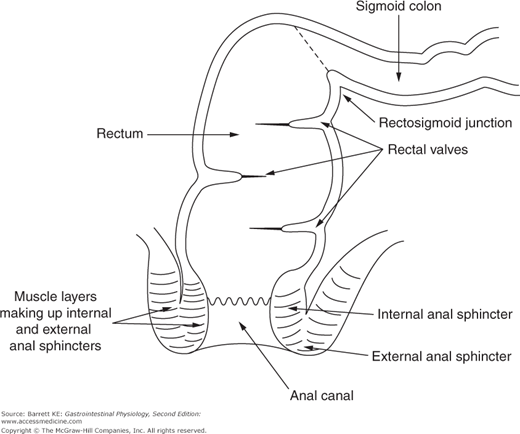
As one moves into the sigmoid colon and rectum, the intestine becomes completely enveloped by longitudinal muscle that is important to the specialized functions of this region, which include serving predominantly as a conduit and participating in defecation. The lumen of the rectum is also partially occluded by transverse folds, again formed by muscular contraction, which act as shelves to retard the passage of fecal material (Figure 9–1). Finally, the most distal portion of the gastrointestinal tract, the anal canal, is a specialized region that contains both smooth and striated muscle in its walls. In this respect, it can be compared with the most proximal gut segment, the esophagus, which is the only other segment of the gastrointestinal system whose motility is governed by both muscle types.
 The primary determinant of motility function in both the small and large intestines derives from the activity of intrinsic neural circuits. The number of intrinsic nerves vastly exceeds that of extrinsic inputs, and the role of the latter is normally felt to be restricted largely to modulating motility patterns established by the “little brain” of the enteric nervous system, rather than independently initiating muscle activity. Enteric nerves contain a variety of neurotransmitters and may be responsible for smooth muscle contraction or relaxation. The major stimulatory neurotransmitters include acetylcholine (ACh), neurokinin A and substance P, whereas the inhibitory nerves express vasoactive intestinal polypeptide (VIP) and also produce nitric oxide on activation. There is also an abundant supply of sensory afferents that respond to the physicochemical characteristics of the luminal contents. Additional information about the coding of enteric nerves is provided in Chapter 2.
The primary determinant of motility function in both the small and large intestines derives from the activity of intrinsic neural circuits. The number of intrinsic nerves vastly exceeds that of extrinsic inputs, and the role of the latter is normally felt to be restricted largely to modulating motility patterns established by the “little brain” of the enteric nervous system, rather than independently initiating muscle activity. Enteric nerves contain a variety of neurotransmitters and may be responsible for smooth muscle contraction or relaxation. The major stimulatory neurotransmitters include acetylcholine (ACh), neurokinin A and substance P, whereas the inhibitory nerves express vasoactive intestinal polypeptide (VIP) and also produce nitric oxide on activation. There is also an abundant supply of sensory afferents that respond to the physicochemical characteristics of the luminal contents. Additional information about the coding of enteric nerves is provided in Chapter 2.
Modulatory influences of extrinsic nerves derive from a variety of sources depending on the intestinal segment in question. The vagus (parasympathetic) and splanchnic (sympathetic) nerves innervate the small intestine, ileocecal valve, and proximal colon. The pelvic nerves, on the other hand, are the conduits of extrinsic input to the remainder of the colon and the internal anal sphincter. Finally, the pudendal nerves provide input from the sacral region of the spinal cord to the external anal sphincter and the muscle layers of the pelvic floor. In fact, unlike the other gastrointestinal regions discussed earlier, voluntary input to these latter structures is vital to their function. The ability to contract the external anal sphincter and pelvic floor muscles, a behavior learned during toilet training, allows us to defer defecation until a time when it is socially convenient.
 As alluded to earlier, the passage of contents along the length of the small intestine and colon is also regulated by sphincters. The ileocecal valve, or junction, is a localized zone of high pressure that cannot be abolished by neurotoxins, and which reflects the activity of the circular muscle layer. Unlike sphincters that we have encountered more proximally in the gastrointestinal tract, the primary function of the ileocecal valve does not appear to relate to the control of delivery of luminal contents to the next segment downstream, at least under normal conditions. Rather, the critical function of this valve is apparently to limit reflux of colonic contents into the ileum. This function is vital in maintaining the relative sterility of the small intestine, and injury to or dysfunction of this region can result in the overgrowth of bacteria in the small intestine. Indeed, the ileocecal valve contracts in response to colonic distension, a response that clearly can contribute to limiting reflux of the fecal stream. This reflex is mediated by sympathetic input from the splanchnic nerve. Under certain conditions, however, the ileocecal valve may also retard the aboral passage of the intestinal contents. This is thought to occur primarily under conditions of massively increased flow through this region, such as is seen in secretory diarrheal diseases that target the small intestine.
As alluded to earlier, the passage of contents along the length of the small intestine and colon is also regulated by sphincters. The ileocecal valve, or junction, is a localized zone of high pressure that cannot be abolished by neurotoxins, and which reflects the activity of the circular muscle layer. Unlike sphincters that we have encountered more proximally in the gastrointestinal tract, the primary function of the ileocecal valve does not appear to relate to the control of delivery of luminal contents to the next segment downstream, at least under normal conditions. Rather, the critical function of this valve is apparently to limit reflux of colonic contents into the ileum. This function is vital in maintaining the relative sterility of the small intestine, and injury to or dysfunction of this region can result in the overgrowth of bacteria in the small intestine. Indeed, the ileocecal valve contracts in response to colonic distension, a response that clearly can contribute to limiting reflux of the fecal stream. This reflex is mediated by sympathetic input from the splanchnic nerve. Under certain conditions, however, the ileocecal valve may also retard the aboral passage of the intestinal contents. This is thought to occur primarily under conditions of massively increased flow through this region, such as is seen in secretory diarrheal diseases that target the small intestine.
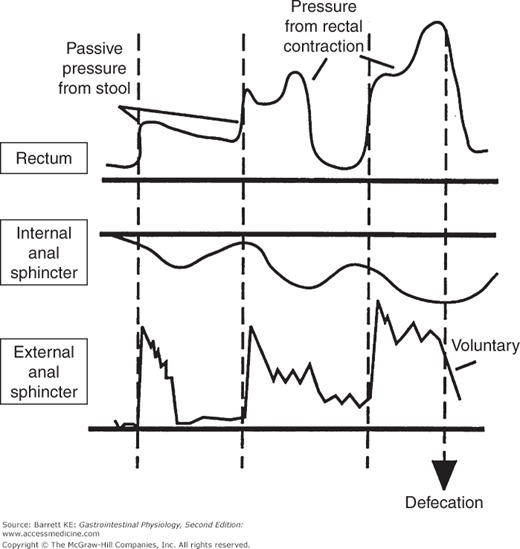
 The elimination of waste matter from the colon is under the control of the internal and external anal sphincters. The internal anal sphincter consists of a thickened band of gastrointestinal circular muscle (Figure 9–1). This sphincter supplies approximately 70–80% of the tone of the anal canal at rest, and its regulation is entirely autonomous. If the rectum is suddenly distended, the sphincter relaxes in response to release of NO and VIP and then contributes only 40% of anal tone, with the remainder supplied by the external anal sphincter. At the same time, the external anal sphincter pressure is increased. This rectoanal inhibitory reflex, initiated by rectal distension, thus allows for efficient defecation while preventing accidental leakage. After a short period of time, however, the internal anal sphincter accommodates to the new rectal volume and regains its tone, unless defecation can conveniently be completed (Figure 9–2).
The elimination of waste matter from the colon is under the control of the internal and external anal sphincters. The internal anal sphincter consists of a thickened band of gastrointestinal circular muscle (Figure 9–1). This sphincter supplies approximately 70–80% of the tone of the anal canal at rest, and its regulation is entirely autonomous. If the rectum is suddenly distended, the sphincter relaxes in response to release of NO and VIP and then contributes only 40% of anal tone, with the remainder supplied by the external anal sphincter. At the same time, the external anal sphincter pressure is increased. This rectoanal inhibitory reflex, initiated by rectal distension, thus allows for efficient defecation while preventing accidental leakage. After a short period of time, however, the internal anal sphincter accommodates to the new rectal volume and regains its tone, unless defecation can conveniently be completed (Figure 9–2).
Figure 9–2.
Motility of the rectum and anal sphincters in response to rectal filling and during defecation. Note that filling of the rectum with stool causes an initial decrease in internal anal sphincter tone, which is counterbalanced by a reflex contraction of the external anal sphincter. The internal sphincter then accommodates to the new rectal volume, allowing relaxation of the external anal sphincter. Finally, defecation occurs when the external anal sphincter is relaxed voluntarily. (From Chang EB, Sitrin MD, Black DD. Gastrointestinal, Hepatobiliary and Nutritional Physiology. Philadelphia: Lippincott-Raven; 1996.)
The external anal sphincter is comprised of striated muscle, and actually consists of portions of three different muscular structures in the pelvic cavity that wrap around the distal anal canal. Unlike the majority of striated muscles, it maintains a significant resting tone, although at baseline this only accounts for 20–30% of the overall tone of the anal canal. However, it can be contracted voluntarily, and also contracts reflexively in response to a sudden increase in abdominal pressure (such as when coughing or lifting a heavy object).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree