Chapter 9 Integration of Metabolism
I. Hormonal Regulation of Metabolism
A. Overview
1. Hormones act by triggering intracellular signaling pathways leading to
a. Coordinated activation or deactivation of key enzymes (usually by phosphorylation or dephosphorylation)
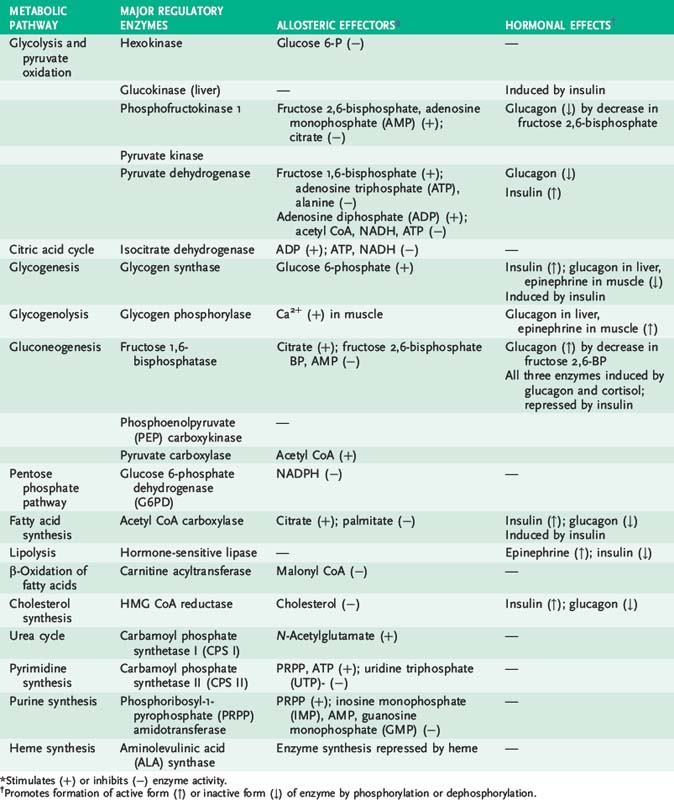
2. Three hormones—insulin, glucagon, and epinephrine—play a critical role in integrating metabolism, especially energy metabolism, in different tissues (Table 9-1).
B. Insulin action
2. Proteolytic cleavage of proinsulin yields C-peptide and active insulin, consisting of disulfide-linked A and B chains.
3. Secretion of insulin is regulated by circulating substrates and hormones.
a. Stimulated by increased blood glucose (most important), increased individual amino acids (e.g., arginine, leucine), and gastrointestinal hormones (e.g., secretin), which are released after ingestion of food
5. The insulin receptor is a tetramer whose cytosolic domain has tyrosine kinase activity for generating second messengers (see Chapter 3).
a. Insulin binding triggers signaling pathways that produce several cellular responses (i.e., post-receptor functions).
b. Increased adipose tissue mass downregulates insulin receptor synthesis, and adipose weight loss upregulates receptor synthesis.
c. Increased glucose uptake by muscle and adipose tissue is prompted by translocation of insulin-sensitive glucose transporter 4 (GLUT4) receptors to the cell surface.
d. Dephosphorylation from insulin action activates energy-storage enzymes (e.g., glycogen synthase) and inactivates energy-mobilizing enzymes (e.g., glycogen phosphorylase).
C. Glucagon and epinephrine action
2. Secretion of glucagon from pancreatic α cells is regulated by circulating substrates and hormones.
3. Secretion of epinephrine from the adrenal medulla is triggered by release of acetylcholine from preganglionic sympathetic nerves in response to stress, prolonged exercise, or trauma.
4. Metabolic actions of glucagon and epinephrine reinforce each other and counteract insulin action.
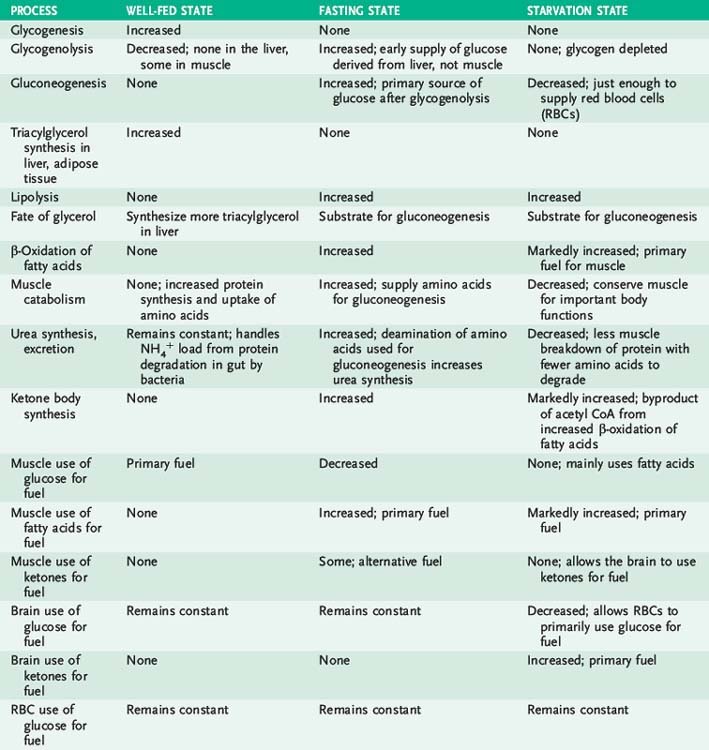
II. The Well-Fed State
A. Overview
1. The metabolic activity of various tissues interacts to store energy when ingested fuel is plentiful (i.e., well-fed state) and to draw on energy stores to maintain blood glucose during fasting or starvation.
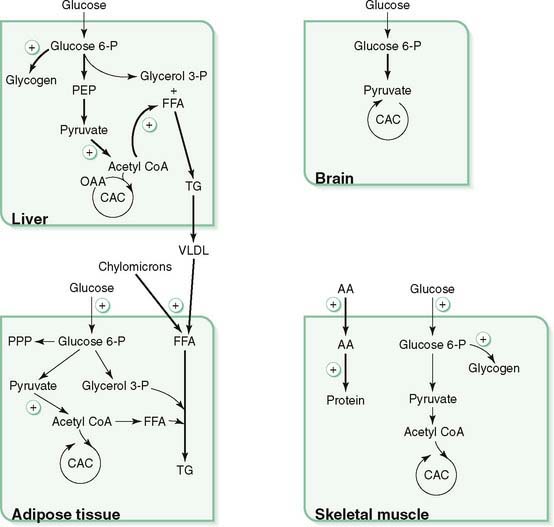
B. Liver metabolism: well-fed state (Fig. 9-1)
1. After a meal, the hepatic portal vein delivers venous blood containing absorbed nutrients (with the exception of long-chain fatty acids) and elevated levels of insulin directly to the liver.
2. Glucokinase traps most of the large glucose influx from the portal vein as glucose 6-phosphate.
a. In contrast to hexokinase, which is present in most tissues, liver glucokinase is active only at high glucose concentrations and is not inhibited by glucose 6-phosphate (see Table 6-1).
3. Active (dephosphorylated) forms of glycogen synthase and pyruvate dehydrogenase are favored by a high insulin-to-glucagon ratio.
4. The oxidative branch of the pentose phosphate pathway provides NADPH, which is required for fatty acid synthesis.
C. Adipose tissue metabolism: well-fed state
2. Increased glucose uptake by insulin-sensitive GLUT4 provides glycerol 3-phosphate for esterification of free fatty acids and storage of triacylglycerol.
3. Increased lipoprotein lipase activity promotes release and uptake of free fatty acids from chylomicrons and VLDL.
D. Muscle metabolism: well-fed state
1. High insulin increases glucose uptake by insulin-sensitive GLUT4 and activation of glycogen synthase leading to the formation of glycogen.