CHAPTER 37 Inner ear
The inner ear contains the organ of hearing, the cochlea, and the organs of balance, the utricle, saccule and semicircular canals. It consists of the bony (osseous) labyrinth, a series of interlinked cavities in the petrous temporal bone, and the membranous labyrinth of interconnected membranous sacs and ducts that lie within the bony labyrinth. The gap between the internal wall of the bony labyrinth and the external surface of the membranous labyrinth is filled with perilymph, a clear fluid with an ionic composition similar to that of other extracellular fluids, i.e. low in potassium ions and high in sodium and calcium. The membranous labyrinth contains endolymph, a fluid with an ionic composition more like that of cytosol, i.e. high in potassium ions and low in sodium and calcium. Moreover, the endolymphatic compartment is approximately 80 mV more positive than the perilymphatic compartment. These differences in ionic composition and potential are essential to the primary function of the inner ear because they provide the driving force for mechanotransduction, the process by which sensory hair cells convert the vibrations set up in the inner ear fluids by head or sound movements into electrical signals that are transmitted via the vestibulocochlear nerve to the vestibular and cochlear nuclei respectively in the brain stem.
The disarticulated temporal bone is described in detail in Chapter 36. The internal acoustic meatus and bony labyrinth are described here.
OSSEOUS (BONY) LABYRINTH
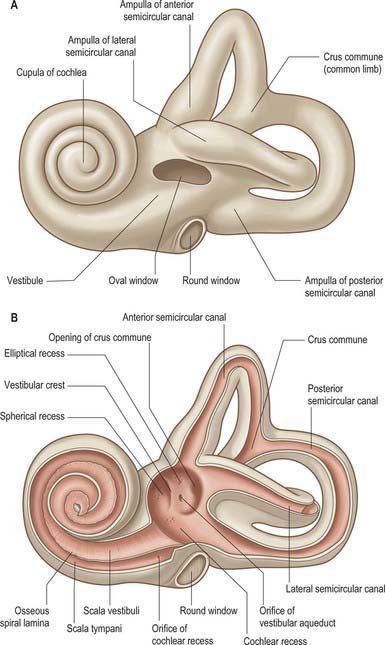
The bony labyrinth lies within the petrous part of the temporal bone. It consists of the vestibule, semicircular canals and cochlea, which are all cavities lined by periosteum and which contain the membranous labyrinth (Fig. 37.1; see Fig. 37.3A). The bone is denser and harder than that of the other parts of the petrous bone, and it is therefore possible, particularly in young skulls, to dissect the bony labyrinth out from the petrous temporal bone.
VESTIBULE
The vestibule is the central part of the bony labyrinth and lies medial to the tympanic cavity, posterior to the cochlea and anterior to the semicircular canals (Figs 37.1 and 37.2). It is somewhat ovoid in shape but flattened transversely, and (on average) measures 5 mm from front to back and vertically, and 3 mm across. In its lateral wall is the opening of the oval window (fenestra vestibuli) into which the base of the stapes inserts, and to which the base of the stapes is attached by an anular ligament. Anteriorly, on the medial wall, is a small spherical recess that contains the saccule; it is perforated by several minute holes, the macula cribrosa media, which transmit fine branches of the vestibular nerve to the saccule. Behind the recess is an oblique vestibular crest, the anterior end of which forms the vestibular pyramid. This crest divides below to enclose a small depression, the cochlear recess, which is perforated by vestibulocochlear fascicles as they pass to the vestibular end of the cochlear duct. Posterosuperior to the vestibular crest, in the roof and medial wall of the vestibule, is the elliptical recess (Fig. 37.1B), which contains the utricle. The pyramid and adjoining part of the elliptical recess are perforated by a number of holes, the macula cribrosa superior. The holes in the pyramid transmit the nerves to the utricle and those in the recess transmit the nerves to the ampullae of the superior and lateral semicircular canals (Fig. 37.1B). The region of the pyramid and elliptical recess corresponds to the superior vestibular area in the internal acoustic meatus (see Fig. 37.4). The vestibular aqueduct opens below the elliptical recess. It reaches the posterior surface of the petrous bone and contains one or more small veins and part of the membranous labyrinth, the endolymphatic duct (see Fig. 37.3A). In the posterior part of the vestibule are the five openings of the semicircular canals; in its anterior wall is an elliptical opening that leads into the scala vestibuli of the cochlea.
SEMICIRCULAR CANALS
The three semicircular canals, anterior (superior), posterior and lateral (horizontal), are located posterosuperior to the vestibule (Fig. 37.1; see Fig. 37.3). They are compressed from side to side and each forms approximately two-thirds of a circle. They are unequal in length, but similar in diameter along their lengths, except where they bear a terminal swelling, an ampulla, which is almost twice the diameter of the canal.
The anterior semicircular canal is 15–20 mm long. It is vertical in orientation and lies transverse to the long axis of the petrous temporal bone under the anterior surface of its arcuate eminence. The eminence may not accurately coincide with this semicircular canal, but may instead be adapted to the occipitotemporal sulcus on the inferior surface of the temporal lobe of the brain. The ampulla at the anterior end of the canal opens into the upper and lateral part of the vestibule. Its other end unites with the upper end of the posterior canal to form the crus commune (common limb), which is 4 mm long, and opens into the medial part of the vestibule.
The two lateral semicircular canals of the two ears are often described as being in the same plane and the anterior canal of one side as being almost parallel with the opposite posterior canal. However, measurements of the angular relations of the planes of the semicircular osseous canals in 10 human skulls led Blanks et al (1975) to suggest that the planes of the three ipsilateral canals are not completely perpendicular to each other. The angles were measured as: horizontal/anterior 111.76 ± 7.55°, anterior/posterior 86.16 ± 4.72°, posterior/horizontal 95.75 ± 4.66°. The planes of similarly orientated canals of the two sides also showed some departure from being parallel: left anterior/right posterior 24.50 ± 7.19°, left posterior/right anterior 23.73 ± 6.71°, left horizontal/right horizontal 19.82 ± 14.93°. The same observers (Curthoys et al 1977) also measured the dimensions and radii of the canals. The means for the radii of the osseous canals were found to be as follows: horizontal 3.25 mm, anterior 3.74 mm, posterior 3.79 mm. The diameters of the osseous canals are 1 mm (minor axis) and 1.4 mm (major axis). The membranous ducts within them are much smaller, but are also elliptical in transverse section, and have major and minor axes of 0.23 and 0.46 mm (see Fig. 37.3B). Representative means for ampullary dimensions are as follows: length 1.94 mm, height 1.55 mm. Phylogenetic studies suggest that the arc sizes of the semicircular canals in humans and other primates may be functionally linked to sensory control of body movements. The angulation and dimensions of the canals may be related to locomotor behaviour and possibly to agility, or more specifically to the frequency spectra of natural head movements (see review by Spoor & Zonneveld 1998).
COCHLEA
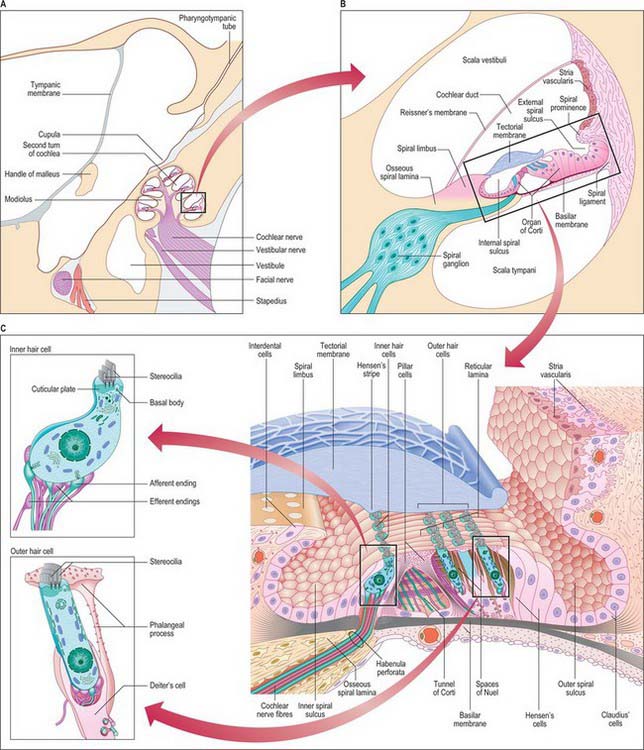
The cochlea (from the Greek cochlos for snail) is the most anterior part of the labyrinth, lying in front of the vestibule (Figs 37.1 and 37.2A; see Fig. 37.3A). It is 5 mm from base to apex, and 9 mm across its base. Its apex, or cupula, points towards the anterosuperior area of the medial wall of the tympanic cavity (Fig. 37.2A). Its base faces the bottom of the internal acoustic meatus and is perforated by numerous apertures for the cochlear nerve. The cochlea has a conical central bony core, the modiolus, and a spiral canal runs around it. A delicate osseous spiral lamina (or ledge) projects from the modiolus, partially dividing the canal (Fig. 37.2B). Within this bony spiral lies the membranous cochlear duct, attached to the modiolus at one edge and to the outer cochlear wall by its other edge. There are therefore three longitudinal channels within the cochlea. The middle canal (the cochlear duct or scala media) is blind, and ends at the apex of the cochlea; its flanking channels communicate with each other at the modiolar apex at a narrow slit, the helicotrema (Fig. 37.3A). Two elastic membranes form the upper and lower bounds of the scala media. One is Reissner’s membrane, the thin vestibular membrane that separates the scala media from the scala vestibuli. The other is the basilar membrane, which forms the partition between the scala media and the scala tympani. The organ of Corti, the sensory epithelium underlying hearing, sits on the inner surface of the basilar membrane. At the base of the scala vestibuli is the oval window (fenestra vestibuli), which leads onto the vestibular cavity but is sealed by the footplate of the stapes. The scala tympani is separated from the tympanic cavity by the secondary tympanic membrane at the round window (fenestra cochleae). The central cochlear core, the modiolus, has a broad base near the lateral end of the internal acoustic meatus, where it corresponds to the spiral tract (tractus spiralis foraminosus). There are several openings in this area for the fascicles of the cochlear nerve: those for the first 1½ turns run through the small holes of the spiral tract, and those for the apical turn run through the hole that forms the centre of the tract. Canals from the spiral tract go through the modiolus and open in a spiral sequence into the base of the osseous spiral lamina. Here the small canals enlarge and fuse to form Rosenthal’s canal, a spiral canal in the modiolus which follows the course of the osseous spiral lamina and contains the spiral ganglion (Fig. 37.2B). The main tract continues through the centre of the modiolus to the cochlear apex.
The osseous or primary spiral lamina is a ledge that projects from the modiolus into the osseous canal like the thread of a screw (Fig. 37.2B). It is attached to the inner edge of the basilar membrane and ends in a hook-shaped hamulus at the cochlear apex, partly bounding the helicotrema (Fig. 37.3A), which is an opening connecting the scala tympani and scala vestibuli. From Rosenthal’s canal, many tiny canals, the habenula perforata, radiate through the osseous lamina to its rim; they carry fascicles of the cochlear nerve to the organ of Corti. A secondary spiral lamina projects inwards from the outer cochlear wall towards the osseous spiral lamina and is attached to the outer edge of the basilar membrane. It is most prominent in the lower part of the first turn: the gap between the two laminae increases progressively towards the cochlear apex, which means that the basilar membrane is wider at the apex of the cochlea than at the base.
MICROSTRUCTURE OF THE BONY LABYRINTH
The wall of the bony labyrinth is lined by fibroblast-like perilymphatic cells and extracellular fibres (Fig. 37.3B). The morphology of the cells varies in different parts of the labyrinth. Where the perilymphatic space is narrow, as in the cochlear aqueduct, the cells are reticular or stellate in form, and give off sheet-like cytoplasmic extensions that cross the space. Where the space is wider, as in the scalae vestibuli and tympani of the cochlea and much of the vestibule, the perilymphatic cells on the periosteum and the external surface of the membranous labyrinth are extremely flat, and resemble a squamous epithelium. Elsewhere, on parts of the perilymphatic surface of the basilar membrane, the cells are cuboidal. Bundles of collagen fibres are closely related to the periosteal and labyrinthine aspects of these cells.
Recent evidence suggests that micropores or canaliculi (canaliculi perforantes) (0.2–23.0 μm diameter) are more widely distributed within the bony surfaces lining the perilymphatic space than was previously suspected: they are numerous in the peripheral and modiolar portions of the osseous spiral lamina and the floor of the scala tympani, but sparse in the osseous wall of the scala vestibuli. The proposal that these canaliculi normally provide an extensive fluid communication channel between the scala tympani and the spiral canal of the cochlea could have implications not only for novel drug-based cochlear therapies delivered via the scala tympani and the delivery of stem cells or appropriate cell lines into the deafened cochlea, but also for the design of implanted perimodiolar electrode arrays (Shepherd & Colreavy 2004). (For further reading about the changes in the inner ear that are induced by implanted cochlear electrodes, both acute and long term, see Kiefer et al 2006.)
Composition of inner ear fluids
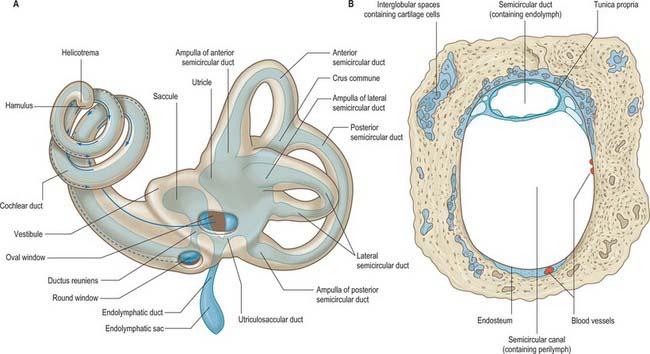
The space between the bony and membranous labyrinths is filled with perilymph (Fig. 37.3B). The membranous labyrinth is filled with endolymph, a fluid produced by the marginal cells of the stria vascularis and the dark cells of the vestibule (see review by Wangemann & Schacht 1996) (Fig. 37.2B). Whatever their relative contributions, endolymph probably circulates in the labyrinth; it enters the endolymphatic sac, where it is transferred into the adjacent vascular plexus via the specialized epithelium of the sac. Pinocytotic removal of fluid may also occur in other labyrinthine regions.
Perilymph was initially considered to be an ultrafiltrate of plasma because of its low protein content. However, it more closely resembles cerebrospinal fluid in ionic composition, particularly in the scala tympani. Its composition is not precisely the same in both cochlear scalae: concentrations of potassium, glucose, amino acids and proteins are greater in the scala vestibuli. This has led to the suggestion that perilymph in the scala vestibuli is derived from plasma via the endothelial boundary of the cochlear blood vessels, whereas the perilymph in the scala tympani contains some cerebrospinal fluid derived from the subarachnoid spaces via the cochlear canaliculus. However, the lack of significant bulk flow suggests that perilymph homeostasis is predominantly locally regulated. Perilymph contains approximately 5 mM K+, 150 mM Na+, 120 mM Cl− and 1.5 mM Ca2+. Endolymph contains greater K+ (150 mM) and Cl− (130 mM) concentrations and lower Na+ (2 mM) and Ca2+ (20 μM) concentrations than perilymph. The major differences in ionic composition between the two fluids are important for the function of the inner ear. Displacements of the stereociliary bundles of the sensory cells activate relatively non-specific cationic channels in the stereociliary tips which allow an influx of cations, particularly K+ and Ca2+, from the endolymph. Hair cells also possess K+ channels activated by membrane voltage or intracellular Ca2+ concentrations, and these allow efflux of K+ into the perilymph which bathes their basal and lateral membranes. In addition, synaptic transmission at the base and sides of hair cells depends on the influx of Ca2+ from the perilymph through voltage-dependent calcium channels in order to release neurotransmitter.
INTERNAL ACOUSTIC MEATUS
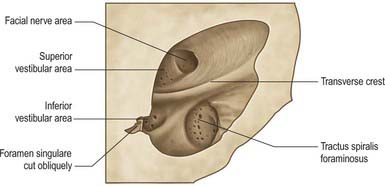
The internal acoustic meatus is separated from the internal ear at its lateral fundus by a vertical plate divided unequally by a transverse crest (Fig. 37.4). The canal for the facial nerve passes above and anterior to the crest. Posterior to the crest, the superior vestibular area contains openings for nerves to the utricle and anterior and lateral semicircular ducts. Below the crest, an anterior cochlear area contains a spiral of small holes, the tractus spiralis foraminosus, which encircles the central cochlear canal. Behind this, the inferior vestibular area contains openings for saccular nerves, and most posteroinferior, a single hole (foramen singulare) admits the nerve to the posterior semicircular duct.
MEMBRANOUS LABYRINTH
The membranous labyrinth is separated from the periosteum by a space that contains perilymph and a web-like network of fine blood vessels (Fig. 37.3). It can be divided into two major regions, the vestibular apparatus and the cochlear duct.
VESTIBULAR APPARATUS
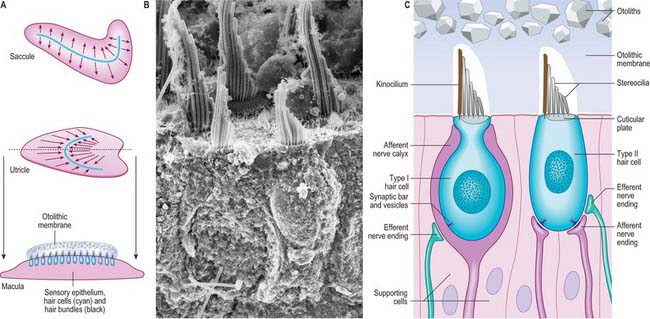
Utricle
The utricle is the larger of the two major vestibular sacs. It is an irregular, oblong, dilated sac that occupies the posterosuperior region of the vestibule (Fig. 37.3A), and contacts the elliptical recess (where it is a blind-ended pouch) and the area inferior to it.
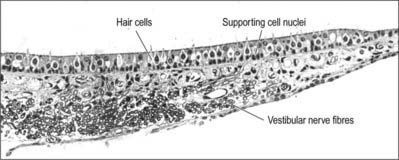
The macula of the utricle (or utriculus) is a specialized area of neurosensory epithelium lining the membranous wall, and is the largest of the vestibular sensory areas (Fig. 37.5). It is triangular or heart-shaped in surface view and lies horizontally with its long axis orientated anteroposteriorly and its sharp angle pointing posteriorly (Fig. 37.6). It is flat except at the anterior edge, where it is gently folded in on itself, and it measures 2.8 mm long by 2.2 mm wide. The mature form of the macula is reached early in development, but in the adult a bulge is often present on the anterolateral border; there is sometimes an indentation at the anteromedial border. The epithelial surface is covered by the otolithic membrane (statoconial membrane), a gelatinous structure in which many small crystals, the otoconia (otoliths, statoliths), are embedded. A curved ridge, the ‘snowdrift line’, runs along the length of the otolithic membrane. It corresponds to a narrow crescent of underlying sensory epithelium termed the striola, 0.13 μm wide. The density of sensory hair cells in this strip of epithelium is 20% less than in the rest of the macula. The striola is convex laterally and runs from the medial aspect of the anterior margin in a posterior direction towards, but not reaching, the posterior pole. The part of the macula medial to the striola is called the pars interna and is slightly larger than the pars externa, which is lateral to it. The significance of this area is that the sensory cells are functionally and anatomically polarized towards it (Fig. 37.6). The macula in each utricle is approximately horizontal when the head is in its normal position. Linear acceleration of the head in any horizontal plane will result in the otolithic membrane lagging behind the movement of the membranous labyrinth as a result of the inertia produced by its mass. The membrane thus maximally stimulates one group of hair cells by deflecting their bundles towards the striola whilst inhibiting others by deflecting their bundles away from it. Hence each horizontal movement of the head will produce a specific pattern of firing in the utricular efferents.
Saccule
The saccule (or sacculus) is a slightly elongated, globular sac lying in the spherical recess near the opening of the scala vestibuli of the cochlea (Fig. 37.6). As already noted, it is connected to the utricle and endolymphatic duct by the utriculosaccular duct, and to the cochlea by the ductus reuniens, which leaves inferiorly to open into the base of the cochlear duct (Fig. 37.3A).
The saccular macula is an almost elliptical structure, 2.6 mm long and 1.2 mm at its widest point. Its long axis is orientated anteroposteriorly but, in contrast to the utricular macula, the saccular macula lies in a vertical plane on the wall of the saccule. Its elliptical shape is very slightly distorted by a small anterosuperior bulge. Like the utricular macula, it is covered by an otolithic (statoconial) membrane and possesses a striola, 0.13 mm wide, which extends along its long axis as an S-shaped strip about which the sensory cells are functionally and anatomically polarized (Fig. 37.6). The part of the macula above the striola is termed the pars interna, and that below it, the pars externa. The operation of the saccule is similar to that of the utricle. However, because of its vertical orientation, the saccule is particularly sensitive to linear acceleration of the head in the vertical plane, and is, therefore, a major gravitational sensor when the head is in an upright position. It is also particularly sensitive to movement along the anteroposterior axis.
Semicircular canals
The lateral, anterior and posterior semicircular ducts follow the course of their osseous canals. Throughout most of their length they are securely attached, by much of their circumference, to the osseous walls. They are approximately one-quarter of the diameter of their osseous canals (Fig. 37.3B). The medial ends of the anterior and posterior canals fuse to form a single common duct, the crus commune, before entering the utricle. The lateral end of each canal is dilated to form an ampulla, which lies within the ampulla of the osseous canal. The short segment of duct between the ampullae and utricle is the crus ampullaris.
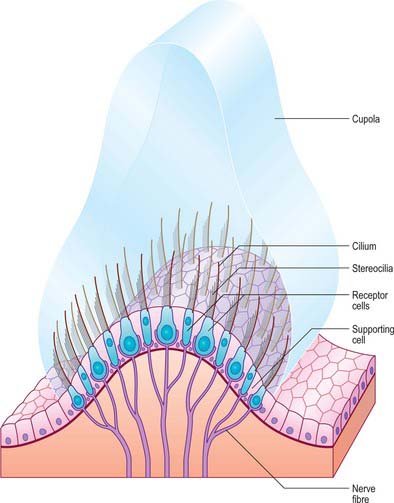
The membranous wall of each ampulla contains a transverse elevation (septum transversum) on the central region of which is a sensory area, the ampullary crest (crista). This is a saddle-shaped ridge that lies transversely across the duct. It is broadly concave on its free edge along most of its length and has a concave gutter (planum semilunatum) at either end between the ridge and the duct wall. Sectioned across the ridge, the crests of the lateral and anterior semicircular canals have smoothly rounded corners; the posterior crest is more angular. A vertical plate of gelatinous extracellular material, the cupula, is attached along the free edge of the crest (Fig. 37.7). It projects far into the lumen of the ampulla so that movements of endolymph within the duct readily deflect the cupula and therefore also the stereocilia of the sensory cells that are inserted into its base. The three semicircular canals thus detect angular accelerations during tilting or turning movements of the head in any direction.
Microstructure of the vestibular system
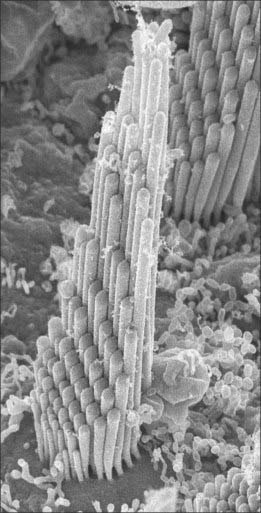
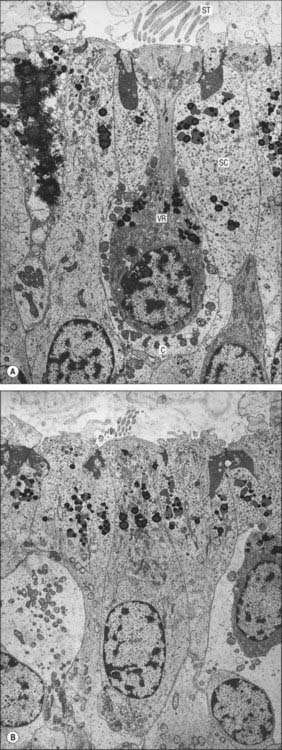
Type I vestibular sensory cells measure 25 μm in length, with a free surface of 6–7 μm in diameter. The basal part of the cell does not reach the basal lamina of the epithelium. Each cell is typically bottle-shaped, with a narrow neck and a rather broad, rounded basal portion containing the nucleus (Fig. 37.6). The apical surface is characterized by 30–50 stereocilia (large, regularly-arranged, modified microvilli about 0.25 μm across) and a single kinocilium (with the typical ‘9 + 2′ arrangement of microtubules characteristic of true cilia). The kinocilium is considerably longer than the stereocilia, and may attain 40 μm, whereas the stereocilia are of graded lengths. They are characteristically arranged in regular rows behind the kinocilium in descending order of height, the longest being next to the kinocilium (Fig. 37.8). The kinocilium emerges basally from a typical basal body, with a centriole immediately beneath it. Close to the inner surface of their basal two-thirds, every cell contains numerous synaptic ribbons with associated synaptic vesicles (see p. 45). The postsynaptic surface of an afferent nerve ending encloses the greater part of the sensory cell body in the form of a cup (chalice or calyx). Efferent nerve fibres make synapses with the external surface of the calyx, rather than directly with the sensory cell.
There is much greater variation in the sizes of type II sensory cells (Fig. 37.9). Some are up to 45 μm long, and almost span the entire thickness of the sensory epithelium, whereas others are shorter than type I cells. They are mostly cylindrical, but otherwise resemble type I cells in their contents and the presence of an apical kinocilium and stereocilia. However, their kinocilia and stereocilia tend to be shorter and less variable in length. The most striking difference between type I and II cells is their efferent nerve terminals: type II cells receive several efferent nerve boutons containing a mixture of small clear and dense-core vesicles around their bases. Afferent endings are small expansions rather than chalices.
Each sensory cell is structurally and functionally polarized (Fig. 37.6). Deflection of the hair bundle towards the kinocilium results in depolarization of the hair cell, and increases the rate of neurotransmitter release from its base. Deflection away from the kinocilium hyperpolarizes the hair cell and reduces the release of neurotransmitter. The hair cells have specific orientations within each sensory organ. In the maculae, they are arranged symmetrically on either side of the striola. In the utricle, the kinocilia are positioned on the side of the sensory cell nearest to the striola. In the saccule, they are furthest from it. In the ampullary crests, the cells are orientated with their rows of stereocilia at right angles to the long axis of the semicircular duct. In the lateral crest the kinocilia are on the side towards the utricle, whereas in the anterior and posterior crests they are away from it. These different arrangements are important functionally, because any given acceleration of the head maximally depolarizes one group of hair cells and maximally inhibits a complementary set, thus providing a unique representation of the magnitude and orientation of any movement (for further details, see Furness 2002).
The type I and II sensory cells are set within a matrix of supporting cells that reach from the base of the epithelium to its surface, and form rosettes round the sensory cells, as seen in surface view. Although their form is irregular, they can easily be recognized by the position of their nuclei, which tend to lie below the level of sensory cell nuclei and just above the basal lamina (Fig. 37.5). The apices of the supporting cells are attached by tight junctions to neighbouring cells to produce the reticular lamina, a composite layer which forms a plate that is relatively impermeable to cations other than via the mechanosensitive transduction channels of the hair cells.
The otolithic membrane is a layer of extracellular material with a complex structure. It can be divided into two strata. The external layer is composed of otoliths or otoconia, which are barrel-shaped crystals of calcium carbonate with angular ends, up to 30 μm long, and heterogeneous in distribution (Fig. 37.6
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree