X Inactivation, Dosage Compensation, and the Expression of X-Linked Genes
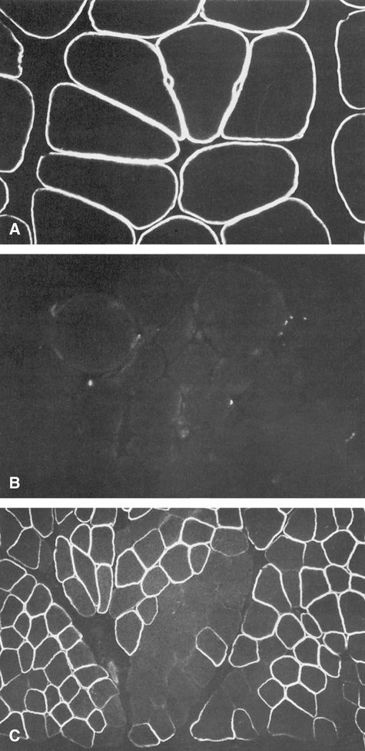
As introduced in Chapters 3 and 6, X inactivation is a normal physiological process in which most of the genes on one of the two X chromosomes in normal females, but not the genes on the single X chromosome in males, are inactivated in somatic cells, thus equalizing the expression of most X-linked genes in the two sexes. The clinical relevance of X inactivation in X-linked diseases is profound. It leads to females having two cell populations, which express alleles of X-linked genes on one or the other of the two X chromosomes (see Fig. 3-13 and further discussion in Chapter 6). These two cell populations are thus genetically identical but functionally distinct, and both cell populations in human females can be readily detected for some disorders. For example, in Duchenne muscular dystrophy (Case 14), female carriers exhibit typical mosaic expression of their dystrophin immunostaining (Fig. 7-11). Depending on the pattern of random X inactivation of the two X chromosomes, two female heterozygotes for an X-linked disease may have very different clinical presentations because they differ in the proportion of cells that have the mutant allele on the active X in a relevant tissue (as seen in manifesting heterozygotes, as described later).
Recessive and Dominant Inheritance of X-Linked Disorders
As mentioned earlier in this chapter, the use of the terms dominant and recessive is somewhat different in X-linked conditions than we just saw for autosomal disorders. So-called X-linked dominant and recessive patterns of inheritance are typically distinguished on the basis of the phenotype in heterozygous females. Some X-linked phenotypes are consistently apparent clinically, at least to some degree, in carriers and are thus referred to as dominant, whereas others typically are not and are considered to be recessive. The difficulty in classifying an X-linked disorder as dominant or recessive arises because females who are heterozygous for the same mutant allele in a family may or may not demonstrate the disease, depending on the pattern of random X inactivation and the proportion of the cells in pertinent tissues that have the mutant allele on the active or inactive X.
Nearly a third of X-linked disorders are penetrant in some but not all female heterozygotes and cannot be classified as either dominant or recessive. Even for disorders that can be so classified, they show incomplete penetrance that varies as a function of X inactivation patterns, not inheritance patterns. Because clinical expression of an X-linked condition does not depend strictly on the particular gene involved or even the particular mutation in the same family, some geneticists have recommended dispensing altogether with the terms recessive and dominant for X-linked disorders. Be that as it may, the terms are widely applied to X-linked disorders, and we will continue to use them, recognizing that they describe extremes of a continuum of penetrance and expressivity in female carriers of X-linked diseases.
X-Linked Recessive Inheritance
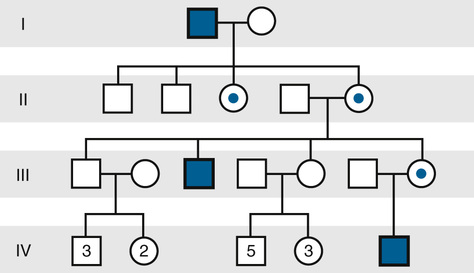
The inheritance of X-linked recessive phenotypes follows a well-defined and easily recognized pattern (Fig. 7-12 and Box). An X-linked recessive mutation is expressed phenotypically in all males who receive it, and, consequently, X-linked recessive disorders are generally restricted to males.
Hemophilia A is a classic X-linked recessive disorder in which the blood fails to clot normally because of a deficiency of factor VIII, a protein in the clotting cascade (Case 21). The hereditary nature of hemophilia and even its pattern of transmission have been recognized since ancient times, and the condition became known as the “royal hemophilia” because of its occurrence among descendants of Britain’s Queen Victoria, who was a carrier.
As in the earlier discussion, suppose Xh represents the mutant factor VIII allele causing hemophilia A, and XH represents the normal allele. If a male with hemophilia mates with a normal female, all the sons receive their father’s Y chromosome and a maternal X and are unaffected, but all the daughters receive the paternal X chromosome with its hemophilia allele and are obligate carriers. If a daughter of the affected male mates with an unaffected male, four genotypes are possible in the progeny, with equal probabilities:
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree