Inflammatory Bowel Disease-Associated Neoplasia
Julianne K. Purdy, MD
Key Facts
Macroscopic Features
Polypoid dysplastic lesion/DALM
Adenoma-like: Endoscopically resectable, dome-shaped, sharp borders
Non-adenoma-like: Not endoscopically resectable, sessile/plaque-like, indistinct margins
Microscopic Pathology
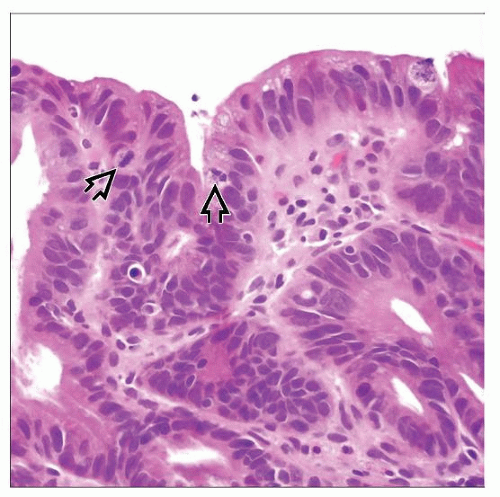
Low-grade dysplasia
Nuclei uniformly enlarged and hyperchromatic; nuclear stratification in basal 1/2 of cells
Minor glandular crowding; no cribriform architecture
High-grade dysplasia
Ovoid hyperchromatic nuclei; high N:C ratio; complete loss of nuclear polarity
Crypt branching/budding; cribriform architecture
Polypoid dysplastic lesion/DALM
Cannot histologically distinguish sporadic adenoma and IBD-related polypoid dysplasia
Non-adenoma-like DALM: Adjacent flat dysplasia; more asymmetry/architectural variability
Diagnose as polypoid low- or high-grade dysplasia; endoscopist decides management
Confirm dysplasia with GI pathology expert
Top Differential Diagnoses
Regeneration within inflammation
Surface maturation (goblet cells), nuclear stratification only at bases of crypts
Vesicular nuclei, prominent nucleoli, abundant eosinophilic cytoplasm
No crypt budding/branching; no cribriform architecture
TERMINOLOGY
Synonyms
Dysplasia
Definitions
Dysplasia or carcinoma associated with longstanding inflammatory bowel disease (IBD)
Dysplasia-associated lesion or mass (DALM) = polypoid dysplastic lesion
Term 1st used to describe sessile plaque-like lesions that were not endoscopically resectable
Term “adenoma-like” DALM used to describe discrete endoscopically resectable polyps identical to sporadic adenomas
ETIOLOGY/PATHOGENESIS
Genetic/Familial Link
Colorectal cancer (CRC) in 1st degree relative: ≥ 2x higher risk of CRC Inflammation → Genomic Instability
> 10 years of disease & pancolitis: Highest risk for CRC
Risk in Crohn disease (CD) similar to that for ulcerative colitis (UC) with comparable duration/extent
Left-sided UC: Increased CRC risk after 15-20 years of disease (˜ 10 years later than if pancolitis)
Ulcerative proctosigmoiditis: No increased risk
Response to inflammation overwhelmed → widespread genomic instability
Emergence of neoplastic clones → dysplasia/carcinoma
Silent genetic damage to mucosa before dysplasia
70-85% of CRC in IBD: Chromosomal instability pathway
Widespread gains/losses of genomic material, inactivation of tumor suppressor genes
Shared genetic alterations with sporadic CRC; different timing/prevalence
Mutations/loss of p53 common and early in IBD (late in sporadic CRC)
Inactivation of APC gene (common/early in sporadic CRC) & KRAS mutations (common in adenomas) infrequent in IBD
15-30% of CRC in IBD: Microsatellite instability (MSI) pathway
Leads to CRC with MSI, BRAF mutations, or widespread gene promoter hypermethylation
Higher prevalence of MSI in UC-associated CRC than in sporadic CRC (10-15%)
Dysfunction of mismatch repair genes
Activating BRAF mutations: Marker for serrated neoplasia pathway in inflammatory mucosa in IBD; precedes MSI in IBD-related oncogenesis
Methylation of CpG islands in genes in nonneoplastic epithelium of UC patients with high-grade dysplasia (HGD) or CRC
CLINICAL ISSUES
Epidemiology
Incidence
Dysplasia
UC: Prevalence up to 24%; more common than in CD (prevalence 2-16%)
Elevated > flat
In 83-100% of cases of IBD with carcinoma
Dysplasia found distant from carcinoma site in 23-70%
CRC in IBD
Age
Mean age of CRC in IBD patients 40-50 years (10-20 years earlier than CRC in general population)
Median age of IBD patients with polypoid dysplasia within colitis similar to UC patients with CRC
Site
Dysplasia
Distribution: Most often multifocal; can be isolated
UC patients with ileal pouch anal anastomosis (IPAA): Very small risk of dysplasia in ileal pouch; slightly greater risk in transition zone/rectal cuff
Carcinoma
Colon most common site
UC: Rectum and sigmoid colon
CD: 73% in colon (right and rectosigmoid colon)
Frequent synchronous tumors: 12% of IBD-related CRC (3-5% of sporadic CRC)
Synchronous tumor sites: Colon, rectum, anus, and external/internal fistulous tracts
Usually arises in pancolitis
Small intestine: 27% of CD-related adenocarcinoma
Anus (CD)
Presentation
Dysplasia usually asymptomatic
Carcinoma: Most are asymptomatic; rarely symptoms of obstruction
Natural History
Most UC patients do not develop dysplasia or CRC; most with dysplasia do not get CRC
CD patients with only small intestine involvement
Low risk for CRC; case reports in strictures/fistulae
Risk of small intestinal adenocarcinoma 10-12x > general population
Sporadic adenoma/adenoma-like DALM
With complete polypectomy and continued surveillance: Low risk of progression to CRC
IBD patients: Equal risk for sporadic adenomas as general population
Non-adenoma-like DALM: High risk for concurrent/subsequent CRC (> 40% risk of CRC at colectomy)
Flat low-grade dysplasia (LGD)
˜ 20% risk of CRC if immediate colectomy; up to ˜ 1/2 of patients get HGD or CRC within 5 years
CRC risk 9x that of patients without dysplasia
Unifocal LGD as likely to progress to HGD or CRC as multifocal LGD
Associated with underlying low-grade tubuloglandular adenocarcinoma without HGD or DALM
Flat HGD: 20-50% of patients have CRC at colectomy
Indefinite for dysplasia
Risk for HGD or CRC intermediate between that of patients with no dysplasia and flat LGD (9% get HGD/CRC within 5 years)
Dysplasia may regress/be stable for long periods
May develop CRC with only prior LGD or without any prior dysplasia
Treatment
Options, risks, complications
Colonoscopic surveillance
Indefinite for dysplasia: Shorten surveillance interval
After proctocolectomy & IPAA: If chronic pouchitis or history of dysplasia/carcinoma, long-term surveillance
Flat LGD: Shorten surveillance interval or colectomy
Chromoendoscopy: Use dye to improve detection of dysplasia; increases sensitivity/specificity
Endoscopic removal
Polypoid dysplastic lesions: If completely resectable & no flat dysplasia elsewhere, continue surveillance (even if HGD in polyp)
Surgery (colectomy)
Drugs
Prevention: No drug presently effective for primary prevention of CRC in IBD patients
Prognosis
Significantly increased risk for CRC (including synchronous malignancies)
2-3x greater than that of general population
50-60% newly diagnosed IBD-related CRC stage I-II
Stage for stage, prognosis of CRC in IBD patients similar to patients with sporadic CRC
CD patients: CRC has better prognosis than ileal carcinoma
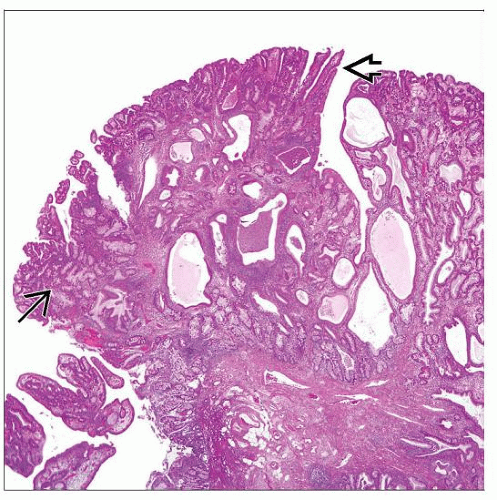
MACROSCOPIC FEATURES
Dysplasia
Flat dysplasia: No endoscopic lesion
Raised: Polypoid dysplastic lesions
May be slight elevation above surrounding mucosa
Recent data suggest most adenoma-like
Adenoma-like DALM
Endoscopically resectable
Dome-shaped, sharp borders, smooth intact surface; no necrosis or fixation to colonic wall
Debate regarding whether IBD-related if within colitis with well-defined stalk (with nondysplastic stalk epithelium)
Non-adenoma-like DALM
Visible lesion within colitis, not endoscopically resectable
Sessile, irregular shape, indistinct margins
Ulceration, velvety patch/plaque, wart-like thickening, stricture, broad-based mass
Carcinoma
Usually flat; difficult to visualize endoscopically
May be minimally raised above surrounding mucosa with indistinct borders
Irregular plaque, villiform carpet-like growth, pedunculated/sessile polyp, stricture, ulcer
MICROSCOPIC PATHOLOGY
Key Descriptors
Histologic features
Dysplasia and carcinoma almost always within diseased mucosa (actively inflamed or healed)
Dysplasia: Negative, indefinite, positive (low or high grade)
Distinction between LGD and HGD based mainly on degree of nuclear stratification & architectural complexity
Number of dysplastic crypts needed to diagnose HGD controversial
How extent of LGD or HGD affects prognosis untested
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree