INTRODUCTION
Surgery and infection are unfortunately intimately intertwined. For the purpose of this chapter, we will differentiate between infections resulting from surgery (surgical site infections) and those resulting from other disease processes but requiring surgical management. Surgical incisions involve a breach of the skin and immune barriers, and can thus be complicated by infection. The term “wound infection” has been replaced by the more accurate term “surgical site infection” (SSI), to emphasize that the infection can occur anywhere within any of the areas accessed surgically (not exclusively at the skin level) and to differentiate it from “traumatic wound infection.”
As opposed to SSI, the term “surgical infection” is used to indicate infections that are unlikely to respond to medical and antimicrobial treatments, and require surgical intervention or management. Common examples include abscesses, empyema, intra-abdominal infections, and necrotizing skin and soft tissue infections. Surgical decision making involves, at its core, the knowledge and experience to determine the timing of surgery and a right balance between surgery and other adjunct therapy such as antibiotic therapy, resuscitative efforts, and nutritional optimization, in order to provide the patient with the best chances to cure the infection with the best overall outcomes.
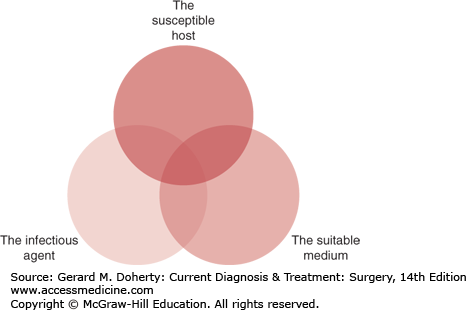
The development of a surgical infection involves a close interplay between three elements:
The degree of contribution of each of these three factors to the eventual occurrence of the infection depends on the individual patient and the specific nature and site of the infection. Whether a given inoculum of bacteria results in an established infection or not depends on the virulence of the bacteria, the strength of the immune and inflammatory host response (eg, chemotaxis, phagocytosis, B- and T-lymphocyte activation), and the amount of blood perfusion and oxygen tension in the medium where the inoculum resides.
Many surgical infections occur in patients with no evidence of decreased immune defenses. However, with the major advances in medicine and health care services over the last century, more immunocompromised patients (eg, transplant, HIV, and diabetic patients) are presenting with infections requiring surgical management or with SSIs following surgical interventions. Table 8–1 delineates a list of patient-related conditions associated with decreased immunity and potential predisposition to surgical infections. The mechanisms by which the listed conditions affect a host’s immunity are diverse in nature. For example, diabetic patients have suboptimal neutrophil adherence, migration and anti-bacterial functions, making them less able to fight an occult infection. Such effect of diabetes on immunity is much more pronounced in patients whose glucose levels are poorly controlled than in those with appropriate and consistent management of their diabetes.
| Advanced age |
| Diabetes mellitus |
| Malnutrition |
| Smoking |
| Obesity |
| Immunosuppressive therapy (eg, posttransplant) |
| Systemic corticosteroid use |
| Peripheral vascular disease |
| Malignancy and anti-neoplastic treatment |
| Concomitant remote site infection |
| Human immunodeficiency virus/acquired immunodeficiency syndrome (HIV/AIDS) |
| Liver failure |
| Renal failure |
Identification of the organism causing the infection is crucial. This is often achieved through the use of Gram stains and cultures of the tissue or purulent material at the site of infection. Staphylococcus aureus is the most common pathogen in SSIs, and its ability to develop resistance to antimicrobial therapy continues to be one of the biggest challenges currently faced by the medical community. Staphylococcus epidermidis is one of the other 33 known species of the genus Staphylococcus, and is usually a skin and mucous membrane nonpathogenic colonizer. Nonetheless, in immunosuppressed individuals and those with indwelling surgical catheters or implants, S epidermidis can cause serious infections requiring treatment. In necrotizing skin and soft tissue infections, the etiology is often polymicrobial with gram-positive, gram-negative, and anaerobic bacteria involved. Monobacterial necrotizing infections can result from either clostridial species (especially Clostridium perfringens) or Streptococcus pyogenes. Streptococci are well known for their ability to invade even minor breaks in the skin and to cause superficial skin and skin structure infections by spreading through connective tissue planes and lymphatic channels. When surgical procedures involve the small or large intestines, gram-negative and anaerobic bacteria are implicated in a significant proportion of the SSIs in addition to the regular gram-positive colonizers. Among these, Escherichia coli, Klebsiella pneumoniae, Enterobacter, and Bacteroides species are the most commonly isolated pathogens. In immunocompromised or critically ill patients, fungi (eg, Candida, Aspergillus, Histoplasma) may result in localized or systemic life-threatening infections. Parasites such as amebas and echinococcus may also cause abscesses in internal organs, especially the liver.
A suitable medium for bacteria consists of a closed space with poor vascular perfusion, low oxygen tension, and low pH. Ischemic and necrotic tissues are perfect examples of such a medium where microorganisms can thrive. The appendix, with its narrow orifice presents a classical example. When an appendicolith blocks the orifice of the appendix, the intraluminal pressure increases and eventually blocks lymphatic and venous outflow. As the vascular perfusion of the appendix diminishes, the tissue oxygen tension decreases, the milieu becomes acidotic, and the appendix becomes ischemic and necrotic. Unless removed, the inflamed appendix and its blocked lumen contain colonic bacteria that will result in an appendiceal infection followed by perforation and a periappendiceal abscess and/or peritonitis.
A foreign body such as a joint prosthesis can become seeded in the event of a systemic infection. As with necrotic tissue, the lack of vascularity decreases the formation of free oxygen radicals within prosthesis and prevents the immune system from readily fighting microorganisms in that area.
The Centers for Disease Control and Prevention (CDC) define SSI as an infection that occurs at or near the surgical incision within 30 postoperative days of the surgical procedure, or within 1 year if an implant is left in place (eg, mesh, heart valve [www.cdc.gov]). The CDC further classifies SSI as
Superficial incisional
Deep incisional
Organ/space SSI
Table 8–2 illustrates the criteria that define each of these three classes of SSIs. It is noteworthy that a skin infection at or around the site of a traumatic wound is classified as a skin and skin structure infection.
Superficial incisional SSI Infection occurs within 30 d after the operative procedure and Involves only skin and subcutaneous tissue of the incision and Patient has at least one of the following:
|
Deep incisional SSI Infection occurs within 30 d after the operative procedure if no implant is left in place or within 1 y if implant is in place and the infection appears to be related to the operative procedure and Involves deep soft tissues (eg, fascial and muscle layers) of the incision and Patient has at least one of the following:
|
Organ/space SSI Infection occurs within 30 d after the operative procedure if no implant is left in place or within 1 y if implant is in place and the infection appears to be related to the operative procedure and Infection involves any part of the body, excluding the skin incision, fascia, or muscle layers, that is opened or manipulated during the operative procedure and Patient has at least one of the following:
|
At least 234 million surgical procedures are performed globally each year, including more than 16 million in the United States alone; SSIs develop in 2%-5% of these patients. In this surgical patient population, SSI accounts for up to 38% of nosocomial infections, and as such is considered the most common nosocomial infection in surgical patients. If asymptomatic bacteriuria is excluded, SSI is arguably the most common nosocomial infection overall. The rate of SSI depends on the nature of the surgical procedure performed and the extent of concomitant intraoperative contamination. In an attempt to quantify the inoculum of bacteria typical of certain surgical procedures, a wound classification was developed in 1964 by the National Academy of Sciences (Table 8–3). This classification divides wounds into clean (eg, inguinal hernia repair), clean-contaminated (eg, right hemicolectomy), contaminated (eg, laparotomy for penetrating injury to small intestines), and dirty (eg, laparotomy for peritonitis and intra-abdominal abscesses) wounds. Several studies have emerged since the conception of the classification confirming that, with reasonable risk adjustment, the classes of contamination correlate well with the incidence of postoperative SSI. A more recent 2012 American College of Surgeons-National Surgical Quality Improvement (ACS-NSQIP) study of more than 600,000 patients, suggested that the rates of SSI increases as the degree of contamination increases: 2.58% for clean wounds, 6.67% for clean-contaminated wounds, 8.61% for contaminated wounds, and 11.80% for dirty wounds.
| Clean | These are uninfected operative wounds in which no inflammation is encountered and the respiratory, alimentary, genital, or uninfected urinary tracts are not entered. |
| Clean/contaminated | These are operative wounds in which the respiratory, alimentary, genital, or urinary tract is entered under controlled conditions and without unusual contamination. |
| Contaminated | These include open, fresh, accidental wounds, operations with major breaks in sterile technique or gross spillage from the gastrointestinal tract, and incisions in which acute, nonpurulent inflammation is encountered. |
| Dirty | These include old traumatic wounds with retained devitalized tissue and those that involve existing clinical infection or perforated viscera. |
In addition to the morbidity incurred by patients, the health care and economic burden of SSIs is significant. Multiple studies have consistently documented that SSIs lead to a considerable increase in length of hospital stay (LOS), hospital charges, and societal health services cost. In a 2012 study, SSI occurring after colorectal surgery increased the LOS by 2.8-23.9 days, depending on the nature of the procedure (colon vs rectum resection), the operative approach (open vs laparoscopic), and the depth of the SSI. In another study of cardiac surgery, it was estimated that the total excess cost attributable to SSI is more than $12,000 per patient. This excess cost resulted from the additional procedures (eg, incision and drainage, wound washout, skin grafts), antibiotics and increased hospital LOS. Another study in general and vascular surgery patients estimated the excess cost and LOS at $10,497 and 4.3 days, respectively. In addition, even when SSI patients are successfully discharged, their rate of hospital readmission is at least doubled. Another study using patient-centered surveys and large administrative databases suggested almost a threefold increase in the total cost for patients diagnosed with SSI following discharge from the hospital compared to those who do not develop SSI. The increased costs were accounted for by a significant increase in the use of visiting nurses for wound care, diagnostic imaging, as well as an increase in readmissions to emergency departments and to the hospital.
The etiology of SSI is multifactorial; patient’s comorbidities, the nature of the surgical procedure and technique, and the perioperative environment including infrastructure and processes of care all interact in SSI. It is thought that most SSIs are directly caused by the patient’s endogenous flora at the time of surgery. This is further supported by the fact that, in clean wounds, SSIs are most commonly caused by S aureus or coagulase negative Staphylococcus, both abundantly present on patient’s skin. On the other hand, SSIs following bowel surgery are usually caused by endogenous intestinal flora. When a surgical device or implant is left in place, bacteria can secrete special glycocalyx-based biofilms that shield the offending bacteria from the body’s immune defenses. One of the immediate sequelae of such a phenomenon is the fact that the inoculums needed to cause a SSI in the presence of a foreign body such as a surgical implant or device is typically smaller than the inoculum needed to cause the same infection in the absence of a foreign body.
Despite the belief that most SSIs originate from endogenous microbes, there is convincing literature suggesting that nonendogenous sources are implicated in the occurrence of SSIs as well. Breach of the strict sterile surgical field, undetected surgical gloves’ perforations, increased personnel traffic through the operating room, suboptimal air flow and ventilation in the operating room, and poor surgical technique with excessive tissue injury or tension are some of the many factors implicated in SSI that will be discussed further in the next few sections.
Many risk factors have been implicated as predictors of SSI. Some are directly related to the patient’s health status, immune system, and comorbidities, while others are inherent to the nature of the procedure being performed and therefore are not easily modifiable. A few risk factors reflect suboptimal health care systems’ design and less than reliable processes of care delivery, and their importance relies in the possibility of subjecting them to improvement and optimization strategies.
Several studies have shown that advanced age, diabetes mellitus, obesity, smoking, malnutrition, and immunosuppression (eg, chronic steroid use, HIV/AIDS, posttransplant) are all risk factors for the development of SSI (Table 8–1). Most of these factors are not amenable to immediate preoperative modification or prevention, although long-term control of glucose levels (reflected in better HBA1C levels), smoking cessation, and attempts to improve nutrition may help in the overall performance of the patient perioperatively and may also decrease complications, including SSI.
The nature of the procedure, especially the wound classification (Table 8–3) and the expected inoculum of bacteria into the surgical site are key factors that influence the risk of developing a SSI. For example, the rate of SSIs complicating eye surgery, where the inoculum of bacterial contamination is minimal, is almost non-existent. In comparison, the rate of SSI complicating colorectal surgery remains closer to 20% despite strategies and efforts aimed at prevention. In the early 1990s, the National Healthcare Safety Network (NHSN) risk index, previously called the National Nosocomial Infections Surveillance System (NNIS), was developed in an attempt to better define a priori, a patient’s risk of developing SSI. This index considered the following three factors:
An American Society of Anesthesiologists (ASA) score ≥ 3
A surgical wound classification as either contaminated or dirty
A prolonged duration of procedure over a certain number of hours T, where T depends on the nature of the procedure being performed
The analysis of the CDC data by Culver and colleagues revealed that the rates of SSIs are 1.5%, 2.9%, 6.8%, and 13% in the presence of none, 1, 2, or 3 of the above risk index factors, respectively. More recently, The NHSN has introduced improved SSI risk adjustment models for specific operations based on easily tractable patient risk factors.
Several process- and system-related risk factors have been identified over the years as potential factors contributing for a higher rate of SSI.
In a systematic review of more than 30,000 Medicare patients, Bratzler and colleagues found that less than 56% of patients undergoing surgery received their perioperative antibiotics within the recommended 60-minutes window before incision. In the same study, up to 10% of patients did not receive the appropriate antibiotic regimen that would cover the type of pathogens specific to the procedures the patients were undergoing. The two main components of prophylactic perioperative antibiotics, namely, choice and timing, will be discussed in further detail in the SSI prevention section below.
Operating room staff, including nursing, anesthesia and surgical personnel, are strongly encouraged to immediately report to the surgeon when suspecting a potential breach of the sterile field (eg, inadequate skin preparation, soiled clothes or equipment, glove perforation). In a study of gynecological laparotomies, glove perforations were found to occur in 27 out of 29 laparotomies. The relationship between the rates of SSIs and glove perforation is less than evident when the data is analyzed. In a study of more than 4000 procedures, the rates of SSIs increased with glove perforation only in the absence of appropriate antibiotic prophylaxis.
Preoperative hair removal has been correlated in several studies with a higher rate of SSI, even when the procedure involves the scalp or the patient has abundant hair at the surgical site. A 2006 Cochrane database systematic review and meta-analysis concluded that preoperative shaving increases the rate of SSI by at least twofold; it is noteworthy that there was no difference in the rates of SSI when hair clipping was compared to no hair removal. Therefore, if hair removal is deemed necessary, preoperative hair clipping is preferred to shaving.
The use of “rough” surgical techniques with unnecessary or excessive tissue disruption and trauma, whether resulting from electrocautery, traction or blunt dissection, is believed to lead to higher rates of SSI. Even though most surgeons and common sense suggest the above correlation, strong evidence supporting a cause-effect relationship has not been demonstrated.
It is well established that the burden of airborne bacteria in a closed space is directly related to the number of people in the space, as well as to the number of people entering and exiting a closed space such as the OR. The link between increased traffic through the OR doors and SSIs has been studied mostly in the orthopedic literature, but has not been clearly established. Nonetheless, the CDC has issued guidelines that include a decrease in OR traffic.
Even though hypothermia may protect tissue from ischemia and necrosis by decreasing cellular oxygen consumption, perioperative hypothermia may cause vasoconstriction at the skin level, and therefore decrease oxygen delivery. Two randomized trials attempted to study the effect of hypothermia on the incidence of SSI. The first one found an increased SSI risk with hypothermia in colorectal surgery, while the second failed to find any correlation in cardiac surgery. At present, maintenance of perioperative normothermia is recommended.
Prevention of SSI relies primarily on optimizing the patient’s comorbidities and creating a systematic method to ensure that all modifiable risk factors are addressed. All processes of care proven or arguably thought to prevent SSIs should be executed in all patients at all times. Table 8–4 lists potential measures to minimize the risk of SSI. Although level-one evidence is lacking for many of those measures, inclusion of some or all in a bundle might prove to be a beneficial strategy. The surgical improvement project (SIP) established in 2004 and folded within the surgical care improvement program in 2006 (SCIP) created performance measures out of several of these preventative measures based on best evidence in literature (Table 8–5). A detailed discussion of SCIP is beyond the scope of this text and the reader is referred to the CDC and CMS websites for more details. Perioperative antibiotics and the choice of the skin preparation solution are discussed below.
| Optimizing the patient’s nutritional status |
| Appropriate perioperative antibiotic prophylaxis (choice, dosing, and timing) |
| Adequate glucose level control in diabetic patients |
| Maintenance of perioperative normoglycemia in all patients |
| Maintenance of strict asepsis |
| Preoperative skin preparation with alcohol-based solutions |
| Gentle skin handling and minimization of cautery-related tissue damage |
| Improving ventilation and laminar air flow in the OR |
| Maintenance of perioperative normothermia |
| Avoiding hair removal; clipping instead of shaving, if necessary |
| Perioperative oxygen supplementation |
| Preoperative antibacterial soap showers |
| Antibacterial coated sutures |
| Antibacterial irrigation solutions |
| Operative wound barriers |
| Antibacterial dressings |
| SCIP Infection Measure 1 | Prophylactic antibiotic received within 1 h prior to surgical incision |
| SCIP Infection Measure 2 | Appropriate prophylactic antibiotic selection for the surgical patient |
| SCIP Infection Measure 3 | Prophylactic antibiotic discontinued within 24 h after surgery end time (within 48 h after cardiac surgery end time) |
| SCIP Infection Measure 4 | Blood glucose level at 6 am < 200 mg/dL on postoperative days 1 and 2 in cardiac surgery |
| SCIP Infection Measure 6 | No or appropriate hair removal (clippers not shaving) |
| SCIP Infection Measure 7 | Immediate postoperative normothermia (temperature > 96.8°F within 15 min postoperatively) in colorectal surgery |
The timing of prophylactic antibiotic administration (SCIP 1) and the choice of the appropriate perioperative antibiotic (SCIP 2) are key components in the strategy to prevent SSI. Prophylaxis implies discontinuation of the antibiotic within 24 hours in noncardiac surgery and within 48 hours in cardiac surgery (SCIP 3); this will also prevent side effects of the antibiotic and the emergence of drug resistance. Proper antibiotic dosing based on the patient’s weight and manufacturer’s recommendation for the antibiotic is an important consideration in prophylaxis. In addition, redosing of the antibiotic during surgery based on the antibiotic half-life, amount of fluid administration and blood loss is more important than the continuation of the antibiotic after the wound is closed. Table 8–6 is a practical guide to dosing and time for redosing for commonly used prophylactic antibiotics.
| Antimicrobial | Renal Half-Life (h) | Recommended Infusion Duration | Standard Dose | Weight-Based Dose Recommendationa | Recommended Redosing Interval,b (h) | |
|---|---|---|---|---|---|---|
| Patients With Normal Renal Function | Patients With End-Stage Renal Disease | |||||
| Aztreonam | 1.5-2 | 6 | 3-5 min,c 20-60 mind | 1-2 g iv | 2 g maximum (adults) | 3-5 |
| Ciprofloxacin | 3.5-5 | 5-9 | 60 min | 400 mg iv | 400 mg | 4-10 |
| Cefazolin | 1.2-2.5 | 40-70 | 3-5 min,c 15-60 mind | 1-2 g iv | 20-30 mg/kg (if < 80 kg, use 1 g; if > 80 kg, use 2 g) | 2-5 |
| Cefuroxime | 1-2 | 15-22 | 3-5 min,c 15-60 mind | 1.5 g iv | 50 mg/kg | 3-4 |
| Cefamandole | 0.5-2.1 | 12.3-18 | 3-5 min,c 15-60 mind | 1 g iv | 3-4 | |
| Cefoxitin | 0.5-1.1 | 6.5-23 | 3-5 min,c 15-60 mind | 1-2 g iv | 20-40 mg/kg | 2-3 |
| Cefotetan | 2.8-4.6 | 13-25 | 3-5 min,c 20-60 mind | 1-2 g iv | 20-40 mg/kg | 3-6 |
| Clindamycin | 2-5.1 | 3.5-5f | 10-60 min (do not exceed 30 mg/min) | 600-900 mg iv | If < 10 kg, use at least 37.5 mg; if > 10 kg, use 3-6 mg/kg | 3-6 |
| Erythromycin base | 0.8-3 | 5-6 | NA | 1 g po 19, 18, and 9 h before surgery | 9-13 mg/kg | NA |
| Gentamicin | 2-3 | 50-70 | 30-60 min | 1.5 mg/kg ivg | …g | 3-6 |