Infectious Mononucleosis Lymphadenitis
Definition
Acute lymphadenitis caused by infection with Epstein-Barr virus (EBV).
Synonym
EBV lymphadenitis; infectious mononucleosis.
Etiology
The human herpesvirus family, the Herpesviridae, includes 25 families of viruses but only eight members infect humans: Herpes simplex virus types 1 and 2 [also known as human herpes virus (HHV) 1 and HHV-2], Herpes varicella-zoster (HHV-3), Epstein-Barr virus (HHV-4), cytomegalovirus (HHV-5), HHV-6, HHV-7, and HHV-8 (also known as Kaposi sarcoma herpes virus). All herpes viruses are double-stranded DNA viruses. Despite their structural similarities, the herpes viruses differ significantly in their biochemical and biologic properties and in the human diseases that they can cause.
Infectious mononucleosis is induced by HHV-4, much better known as Epstein-Barr virus (EBV), a γ herpes virus. Epstein-Barr virus has a large genome, a 172–kb linear molecule that encodes for approximately 100 viral proteins (1,2). The viral genome is encased by a nucleocapsid that, in turn, is surrounded by a viral envelope. The virus actively replicates and infects cells in linear form during acute (lytic) infection, but circularizes to become an episome in latently infected cells. Via the gp350 major envelope glycoprotein, EBV can infect B cells via the C3d complement receptor (CD21) (3). The class II major histocompatibility complex molecule is a cofactor necessary for B-cell infection. Both epithelial cells and B cells in the oropharynx can be infected by EBV. The sequence of events has been debated: direct infection of B cells versus epithelial infection followed by B-cell infection. Direct infection of B cells is currently thought to be most likely (2).
Epidemiology
Epstein-Barr virus is ubiquitous in nature; however, its spread varies within different human populations (4). Viral spread is via direct contact with human secretions, most often by contact with human saliva. In conditions of inadequate standards of living, EBV infection occurs early in life, in infants and toddlers, and is almost universal. By the age of 3 years, almost 100% of children have positive serologic studies for EBV. The infection may pass unnoticed; however, more than half of the EBV-infected children continue to shed virus and thus disseminate infection. In countries with better living conditions, the number of persons exposed to EBV is less and exposure, when it occurs, tends to occur later in childhood (5). Approximately half of older children exposed to EBV acquire the infection, and this occurs as infectious mononucleosis (IM), an acute, self-limited disease. In the United States, the peak incidence of EBV infection occurs between the ages of 10 and 19 years of age (5,6). In this age group, the incidence is six to eight cases per 1,000 persons per year (5,6). In persons <10 or >30 years of age, the incidence is less than one case per 1,000 person per year. It must be remembered, however, that infectious mononucleosis can develop in the elderly and, in these patients, malignant lymphoma may be suspected clinically (7). Overall, in the United States, approximately 100,000 cases of infectious mononucleosis are reported per year.
Pathogenesis
Epstein-Barr virus is present in the throat of 85% of patients with IM. During acute infection, EBV replicates in B cells, inducing expression of various viral-associated antigens, including viral capsid antigen (VCA), membrane antigen (MA), early antigen (EA), EBV nuclear antigens (EBNA), and lymphocyte-detected membrane antigen (LYDMA). Epstein-Barr virus also induces B-cell activation and proliferation during the first week of illness, and a humoral antibody response to EBV then occurs.
Antibodies against these EBV antigens, absent before the onset of IM, appear during the illness in rising titers and persist for years thereafter (2,4). These antibodies play a role in containing the infection and are used in the serologic diagnosis of IM. In addition, Paul and Bunnell (8) first showed that heterophile antibodies that agglutin sheep erythrocytes are produced in IM patients. Although these heterophil antibodies are not specific for IM, it was subsequently shown that absorption of heterophil antibodies by beef erythrocytes increased specificity for IM, and that horse erythrocytes, rather than sheep erythrocytes, was even more sensitive, providing the basis for the MonoSpot test (9). Subsequent improvements of the MonoSpot test have led to this text still being used for screening, usually followed by testing specifically for EBV antibodies. In immunocompromised patients, serologic testing is less reliable and direct testing for EBV using polymerase chain reaction (PCR)-based tests may be required. PCR testing can provide estimates of viral load as well.
Antibodies against these EBV antigens, absent before the onset of IM, appear during the illness in rising titers and persist for years thereafter (2,4). These antibodies play a role in containing the infection and are used in the serologic diagnosis of IM. In addition, Paul and Bunnell (8) first showed that heterophile antibodies that agglutin sheep erythrocytes are produced in IM patients. Although these heterophil antibodies are not specific for IM, it was subsequently shown that absorption of heterophil antibodies by beef erythrocytes increased specificity for IM, and that horse erythrocytes, rather than sheep erythrocytes, was even more sensitive, providing the basis for the MonoSpot test (9). Subsequent improvements of the MonoSpot test have led to this text still being used for screening, usually followed by testing specifically for EBV antibodies. In immunocompromised patients, serologic testing is less reliable and direct testing for EBV using polymerase chain reaction (PCR)-based tests may be required. PCR testing can provide estimates of viral load as well.
Beginning in the second week of illness, a vigorous cellular immune response develops as the body naturally begins to eliminate EBV-infected cells. This is represented by the activation of natural killer cells and cytolytic T cells (CD8+ > CD4+) (2). Rapid destruction of virus-infected cells by the immune response is associated with release of inflammatory mediators and cytokines that are the primary cause of the symptoms and signs of IM. Eventually, acute EBV infection subsides, but latent infection of memory B cells persists throughout life (2). The persistence of EBV despite the vigorous host immune response indicates that EBV has evolved mechanisms that allow it to elude immune-system attack.
In most patients with IM, the disease resolves in 3 to 4 weeks, although some symptoms such as fatigue can persist longer. Rarely, acute EBV infection can cause death in patients who are apparently immunocompetent, so-called sporadic fatal IM (10) However, in persons with acquired or congenital immunodeficiency syndromes, particularly those involving the T-cell system, EBV infection more often can induce life-threatening diseases or fatal IM, rather than self-limited disease. Relatively common examples of acquired immunodeficiency are patients who have undergone organ transplantation or those with acquired immunodeficiency syndrome (AIDS). In either setting, EBV has a causative role in the increased risk of B-cell lymphoproliferative disorders and unequivocal B-cell lymphomas that occur in these patients (2,11). EBV infection has a similar role in inducing B-cell lymphoproliferative disorders in patients with the rare congenital immunodeficiency syndromes.
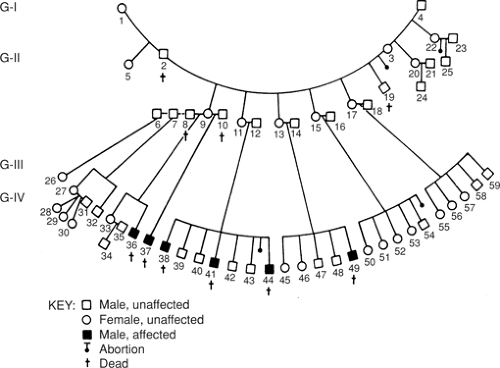
X-linked lymphoproliferative syndrome, also known as Duncan syndrome, is specifically mentioned here because of the high risk of fatal IM that can result after exposure to EBV infection (12). X-linked lymphoproliferative syndrome (XLP) is a congenital immunodeficiency disease that is estimated to affect 1 in 1 million males (Fig. 9.1). These males, usually adolescent boys or young adult men, can normally respond to infection by other viruses, including herpes viruses, but after first exposure to EBV often develop fatal IM (12). The IM affects virtually all lymphoid organs and is similar, clinically and pathologically, to a virus-associated hemophagocytic syndrome. Patients with XLP also have an increased risk of polyclonal or monoclonal B-cell lymphoproliferative disorders, dysgammaglobulinemia, and cytopenias, the latter most often aplastic anemia (12,13). In most patients with XLP, EBV is the trigger leading to the sequelae described, however symptoms can develop in a subset of patients without evidence of EBV infection. Germline mutations of the SAP [signal lymphocyte activation molecular (SLAM)-associated protein] gene, and also known as SH2D1A (SH2 domain 1A), on the X chromosome are present in most patients with XLP (13).
Clinical Syndrome
The incubation period from first exposure to EBV to onset of symptoms can range from 2 to 5 weeks (4). The characteristic triad of symptoms, occurring in over 50% of patients, includes fever, pharyngitis, and cervical or generalized lymphadenopathy (2,4,5,6). A subset of patients has splenomegaly, palatine petechiae, tonsillitis, mild thrombocytopenia, and hepatomegaly. The latter is usually the result of hepatitis with elevated serum liver enzymes; clinical evidence of jaundice is uncommon. Other uncommon or rare complications include splenic rupture, hemolytic anemia, aplastic anemia, skin rash, nephritis, and neurologic diseases (e.g., encephalitis or meningitis).
The peripheral blood leukocyte count typically rises in the second week, usually in the range of 12 to 25 × 109/L, with
lymphocytes and atypical lymphocytes accounting for up to 60% and 10% to 20% of the cells in the differential count, respectively (2,4,5,6). The atypical lymphocytes are predominantly of T-cell lineage and have relatively abundant, pale blue cytoplasm and large nuclei with conspicuous nucleoli (so-called Downey cells) (Figs. 9.2 and 9.3 A,B) (2,6). Neutrophils are usually relatively or absolutely decreased. The bone marrow, although not commonly sampled in IM patients, is often involved, which is indicated by increased numbers of lymphocytes and plasma cells that may form distinct aggregates (Fig. 9.4 A–C). In some patients, peripheral blood findings are minimal or absent, and malaise, fatigue, and palpable lymph nodes are the only manifestations of EBV infection (5,6).
lymphocytes and atypical lymphocytes accounting for up to 60% and 10% to 20% of the cells in the differential count, respectively (2,4,5,6). The atypical lymphocytes are predominantly of T-cell lineage and have relatively abundant, pale blue cytoplasm and large nuclei with conspicuous nucleoli (so-called Downey cells) (Figs. 9.2 and 9.3 A,B) (2,6). Neutrophils are usually relatively or absolutely decreased. The bone marrow, although not commonly sampled in IM patients, is often involved, which is indicated by increased numbers of lymphocytes and plasma cells that may form distinct aggregates (Fig. 9.4 A–C). In some patients, peripheral blood findings are minimal or absent, and malaise, fatigue, and palpable lymph nodes are the only manifestations of EBV infection (5,6).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree