Infections That Complicate the Insertion of Prosthetic Devices
Ilker Uçkay
Daniel P. Lew
Didier Pittet
The insertion of implants and medical devices is now a common procedure that benefits patients, often in a lifesaving way, who are suffering from a variety of exogenous-acquired (trauma, endocarditis, rheumatic fever, hydrocephalus) or degenerative diseases (arthrosis or arthrosclerosis). A fundamental feature of foreign bodies is their exquisite susceptibility to infection. The inoculum of bacteria necessary to induce such postsurgical infections is extremely low when compared with surgery in the absence of a foreign body (1,2). In addition, bacteria that are often nonpathogenic and normally present as skin commensals (i.e., coagulasenegative staphylococci or Propionibacterium species) are able to cause infections under these conditions (2).
The impact of such infections is profound, because they often result in tissue destruction, serious dysfunction of the prosthetic device, and sometimes systemic dissemination of the pathogen. These infections are very difficult to cure with antimicrobial agents alone and most often necessitate the removal of the device or surgical debridement. In this chapter, we take as paradigms the four most frequent surgical implants: orthopedic implants, vascular devices, cerebrospinal shunts, and breast implants. By analogy, the knowledge of infections associated with these four foreign bodies includes the current knowledge for most surgical implant infections. In the first part, we provide insights into their general pathogenesis, incidence (Table 65-1), microbiology, and prevention. In the second part, we discuss individual aspects of the different implants including clinical presentation, pathogenesis, microbiology, and prevention.
PATHOPHYSIOLOGY
To understand the difficulties underlying foreign body infections, it is necessary to characterize four different problems: (i) biofilms, (ii) local neutrophil dysfunction, (iii) small colony variants (SCV) of staphylococci, and (iv) multiresistant staphylococci.
Biofilms
The biofilm concept is extensively discussed in Chapter 31 and only the most important aspects are summarized here. Fundamental differences exist between surface-adherent bacteria and bacteria present in the fluid (planktonic) phase. It has been suggested that biofilm-enclosed microorganisms escape antibiotic killing because the extracellular material prevents diffusion and bacterial uptake of antimicrobial agents (2,3) (Fig. 65-1). Moreover, microorganisms attached to foreign material and exposed to bactericidal concentrations of antibiotics develop tolerance (i.e., the bacteria become resistant to the lethal effect of the antibiotic) (2,3). In an in vitro model of surface-adherent Staphylococcus aureus growing onto polymethylmethacrylate, it was possible to demonstrate a decreased susceptibility to antimicrobial agents within 4 hours after adherence (3).
Altered Host Defense in the Vicinity of Foreign Material: Neutrophil Dysfunction
Data from a study investigating neutrophils from animals with experimental foreign body infection revealed that cells recovered from the vicinity of the implant produced only a weak respiratory burst and had poor bactericidal activity compared with those collected from the blood of the same animals. This deficiency was due to prior activation of the neutrophils by the foreign material. This phagocytic defect may explain the high susceptibility of foreign bodies
to infection (4). A foreign body reduces the inoculum of S. aureus required to induce infection from more than 100,000 to as few as 100 colony-forming units (5). Additionally, the extracellular slime substance produced by adherent staphylococci has potent immunomodulatory properties (6). Several neutrophil functions appear to be affected. Chemotactic responsiveness is diminished and degranulation of specific granule content is increased. Finally, total joint prostheses may shed ultrahigh molecular weight polyethylene particles, thus impairing the phagocytic abilities of the neutrophil (7).
to infection (4). A foreign body reduces the inoculum of S. aureus required to induce infection from more than 100,000 to as few as 100 colony-forming units (5). Additionally, the extracellular slime substance produced by adherent staphylococci has potent immunomodulatory properties (6). Several neutrophil functions appear to be affected. Chemotactic responsiveness is diminished and degranulation of specific granule content is increased. Finally, total joint prostheses may shed ultrahigh molecular weight polyethylene particles, thus impairing the phagocytic abilities of the neutrophil (7).
TABLE 65-1 Frequency of Foreign Body Infections | |||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| |||||||||||||||||||||||||||||
Staphylococcal Small Colony Variants
Staphylococci are responsible for most infections associated with implants (8). Staphylococcal SCV phenotypes are often found in foreign body infections (9,10). They constitute a subpopulation of bacteria and have atypical colony morphology and unusual biochemical characteristics, thus making them a challenge for clinical microbiologists to identify. Clinically, SCV persist in mammalian cells and are less susceptible to antibiotics, especially aminoglycosides, than their wild-type counterparts and are a cause of recurrent infections (3,11,12). SCV of S. epidermidis, a phenomenon well known in S. aureus (9,10), also exist and can emerge during glycopeptide therapy (13).
Multiresistant Staphylococci
Since implants are particularly prone to infections due to staphylococci, resistance to current antibiotics among these pathogens adds to the heavy burden of disease. The ever-increasing incidence of implant infections due to methicillin-resistant S. aureus (MRSA) (14) and methicillinresistant S. epidermidis (MRSE) (15,16) is a serious challenge. MRSE is already the most commonly encountered variant of S. epidermidis in many healthcare institutions (16,17). With coagulase-negative staphylococci, polyclonal implant infections may occur, and this may be one of the explanations for treatment failure as laboratories do not always perform antibiotic susceptibility testing for all isolates (2). So far, it remains unknown when patients become colonized with MRSE upon hospital admission, but it is probable that it occurs very fast. In a Swedish study, most patients on an orthopedic ward were colonized with MRSE at day 14 of admission (18).
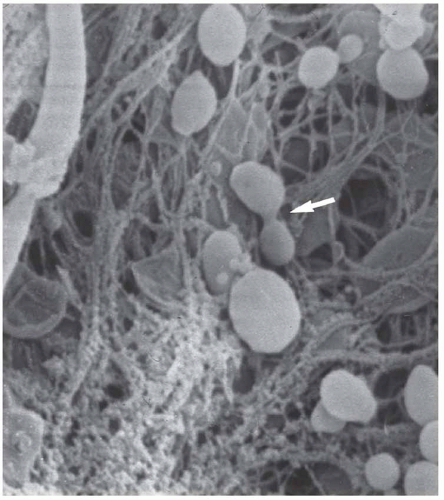 FIGURE 65-1 Biofilm in prosthetic infection. Scanning electron micrograph from infected total hip prosthesis. Cocci-shaped bacteria are shown, partly surrounded by extensive biofilm, amorphous material, and fibrinous elements. In the middle (arrow), the bacterium is dividing. Staphylococcus epidermidis was isolated (2). |
MRSA is usually resistant to many clinically important non-beta-lactam drugs, such as fluoroquinolones and clindamycin that have excellent bone and joint penetration (19). Vancomycin, which is mostly used to treat MRSA infections, has slow bactericidal activity. In this setting, treatment of these infections can be problematic, particularly in the presence of multiple drug intolerance or allergy.
MRSA-related orthopedic implant infections (even with hospital-acquired MRSA) yield a high risk of treatment failure, independent of the clonal microbiological properties and genetic characterization of the isolates. In a recent study including different orthopedic implant material, treatment failure was reported as 35% (20). Failure was nine times more frequent in patients with prosthetic joint infections due to hospital-acquired MRSA than in patients with methicillin-susceptible S. aureus (MSSA) infection (21). However, this remains a controversial issue, and while some reports do not attribute an increased treatment failure (22), others report higher failures in resistant staphylococcal infection (23). Of note, known MRSA skin colonization poorly predicts the pathogen of underlying implant infection due to Staphylococcus species (24).
ORIGIN AND MICROBIOLOGY OF PROSTHETIC INFECTIONS
Surgical site infections (SSI) are discussed in detail in Chapter 21 and only key salient points are provided here. Prostheses become infected by three different routes: (i) through the introduction of microorganisms during the operative procedure (25); (ii) by hematogenous seeding (26) or (iii) by lymphatic spread in the case of adjacent erysipelas. Most SSIs are believed to be acquired during surgery (25), and this is supported by the success of prevention measures targeted on activities inside the operating room and a few reports demonstrating matching strains of pathogens from the surgeon’s fingers and postoperative infection (27). At present, the proportion of SSI really acquired in the operating room versus postoperatively on the ward remains unknown. Similarly, within the subgroup of SSI acquired during surgery, the proportion attributable to the patient himself or herself versus that transmitted by the surgical staff or the operating room environment is also unknown (27). Freshly implanted biomaterial is probably more susceptible to infection. In addition, any factor or
event that delays surgical site healing increases the risk of infection, and ischemic necrosis, infected hematomas, and suture abscesses are common preceding events for joint sepsis (28).
event that delays surgical site healing increases the risk of infection, and ischemic necrosis, infected hematomas, and suture abscesses are common preceding events for joint sepsis (28).
Apart from the setting of cardiac implants, hematogenous seeding probably occurs rarely compared to inoculation during surgery and early postoperative care. The estimated incidence of hematogenous infection among all arthroplasty infections ranges from a low 5.6% (29) to 9% to 10% (27,30). However, in absolute terms, the incidence is quite low. Ainscow et al. (31) detected only three cases of prosthetic joint infections among 1,112 prostheses (2.7 per 1,000) that developed a hematogenous infection. In their prospective arthroplasty cohort, the authors of this chapter detected only seven hematogenous infections among 6,100 arthroplasties (1.1 per 1,000) performed during 1996 to 2008. Arthroplasty patients hospitalized for severe remote infections developed only one hematogenous arthroplasty infection compared with 88 remote infections (27). Any bacteremia can induce an implant infection by hematogenous seeding (27), although there appears to be a higher risk for prosthetic joint infection in the setting of S. aureus bacteremia (32, 33 and 34). The skin is reported to be the most frequent source of infection (25) followed by the genitourinary, respiratory, and gastrointestinal tracts (27). However, this ranking is not fixed and depending on the setting, a gastrointestinal origin can be predominant (27).
Staphylococci (S. aureus and coagulase-negative staphylococci) account for at least 60% of all implant infections (8,35,36) (Table 65-2). Aerobic gram-negative bacteria (37), streptococci, enterococci, and anaerobes cause infection in <25% of cases. Culture-negative arthroplasty infection may occur in 15% of cases (38). Polymicrobial infection is commonly seen as a complication of poor postoperative wound healing (39). Certain comorbidities increase the risk of infection with specific microorganisms. For instance, patients with rheumatoid arthritis are at increased risk for S. aureus infection (40), and Propionibacterium acnes is more commonly encountered in patients with an infected total shoulder arthroplasty (41,42).
TABLE 65-2 Predominant Microorganisms Isolated in Prosthetic Joint Infection | ||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||
PREVENTION OF PROSTHETIC INFECTIONS
Prevention remains the cornerstone of infection control. It is efficacious and certainly more cost-effective than any form of therapy. SSI and preventive measures in general are discussed in detail in Chapter 21. In this section, we highlight special issues regarding the prevention of surgical prostheses infections.
Well-established, modifiable risk factors for SSI are reported in the literature. These include preoperative hair shaving instead of clipping (43), intraoperative hypothermia (44) or hyperglycemia (45), lack of compliance with hand hygiene and surgical antisepsis (46), and suboptimal timing of perioperative antibiotic prophylaxis (47). However, it is probable that no single measure is superior to others in preventing SSI of exogenous origin. This highlights the need for a multimodal approach involving active postdischarge surveillance and measures at every step of patient care: from the operating room to nonnegligent postoperative care (27). An active surveillance program is important for the detection of implant infections (27) and should comprise a minimum length of 1-year follow-up (48). Programs without active postdischarge surveillance readily miss half of all prosthetic infections (27). Multicenter or supranational intervention programs based on guidelines, “bundles,” or safety checklists are likely to be beneficial on a global scale (27).
Antimicrobial Prophylaxis
The benefit of the administration of preoperative antimicrobial agents is largely undisputed. In orthopedic implant surgery, prophylaxis helps to reduce infection rates from 4% to 8% without antibiotics to 1% to 3% according to several trials performed in the 1970s to 1980s (27). Administration of prophylactic antibiotic agents follows some rules. (a) A single intravenous dose of first- or secondgeneration cephalosporins is sufficient for most types of surgery (47, 48, 49, 50 and 51), and an additional benefit of antibiotics in irrigation fluid (47) has not been proven. (b) Timing is of
utmost importance (47,52,53) and prophylaxis should be entirely administered within 1 hour before incision. (c) One dose is sufficient. For operating procedures longer than 4 hours or with significant blood loss, redosing might be justified (47,52). (d) When a tourniquet is used, the entire dose should be administered prior to its inflation (54).
utmost importance (47,52,53) and prophylaxis should be entirely administered within 1 hour before incision. (c) One dose is sufficient. For operating procedures longer than 4 hours or with significant blood loss, redosing might be justified (47,52). (d) When a tourniquet is used, the entire dose should be administered prior to its inflation (54).
A single dose of vancomycin (1 g) is the prophylactic antibiotic of choice for all procedures requiring prophylaxis in patients colonized with MRSA (48,55). Concerning MRSE, a switch to glycopeptide prophylaxis for implant surgery patients is sometimes suggested in the literature. A review of four randomized trials comparing prophylactic teicoplanin versus prophylactic cephalosporin in settings with a high MRSE prevalence identified the same infection rates in both groups (56). This has been confirmed also in a metaanalysis of seven randomized trials for cardiac surgery (57), even though single trials in favor of a general switch to vancomycin prophylaxis in cardiac surgery exist (58). A recent systematic review and economic model of switching from nonglycopeptide to glycopeptide antibiotic prophylaxis for surgery in endemic MRSA settings failed to show increased efficacy in preventing SSI due to methicillin-resistant strains (59). Even for MRSA, there is insufficient evidence to determine whether there is a threshold prevalence to justify a switch to general glycopeptide prophylaxis (59).
Other Prevention Measures
MRSA has the capacity to colonize patients’ skin for several months (62) if not years. These infections represent a failure in quality of care, are costly, and may ultimately compromise patient safety. Prevention is of the utmost importance and the introduction of universal MRSA screening upon hospital admission is seriously debated for this patient population (63,64). However, the results of several outstanding prospective trials in recent years are inconclusive. While some before-after studies (65, 66 and 67) report a benefit, other randomized, crossover design trials (63,68) failed to show a reduction in infection rates (or at least in SSI rates due to MRSA). The debate is not closed. The screening for MSSA nasal colonization and consequent decolonization is equally debated (27). Very recently, a multicenter, double-blind, prospective trial assessing S. aureus carriage at admission by PCR and subsequent nasal and total body decolonization during 5 days showed a significant reduction of hospital-acquired S. aureus. The authors concluded that the number of hospital-acquired surgical site S. aureus infections can be reduced by this strategy. However, results for SSI due to non-S. aureus were not reported (69).
Vaccines provide an attractive conceptual alternative to preventing bacterial infections. A preliminary study with an antistaphylococcal vaccine in hemodialysis patients failed to reduce sepsis due to S. aureus in the first year following vaccination (relative risk reduction: 26%; p = .23) (70), but further research is necessary to explore this possibility.
Aggressive treatment of infection present elsewhere in the body is required before any implant replacement, and all patients need to be informed about this potential complication. Infections should be treated without any delay to allow surgery to be performed. Asymptomatic bacteriuria is probably no risk for subsequent implant infection (26,71).
Many hospitals in resource-rich countries are equipped with relatively expensive vertical or horizontal laminar airflow systems in operating rooms that reduce the bacterial burden in the air (72,73). However, in 2008, a retrospective analysis of the German national nosocomial infection surveillance system showed no benefit of ventilation with laminar airflow and suggested that it was even associated with a significantly higher risk for severe SSI after hip prosthesis (74). Of note, this study has some flaws, such as a lack of data on individual antibiotic prophylaxis, obesity, normothermia, etc., and these findings need confirmation in large-scale studies (75).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


