Infections Associated With Peritoneal Dialysis
John J. Szela
Jeffrey D. Band
HISTORICAL PERSPECTIVES
Although peritoneal dialysis has been used to treat acute renal failure for many years, it has only been over the past 25 to 30 years that peritoneal dialysis has become an alternative to hemodialysis for treatment of chronic renal failure. In 2007, more than 26,000 patients in the United States were maintained on chronic peritoneal dialysis (1). Six percent of the United States dialysis population is undergoing peritoneal dialysis as a form of dialytic therapy.
Two factors have largely contributed to the initial growth of peritoneal dialysis in the treatment of chronic renal failure. First, the introduction of an implantable, cuffed, indwelling silicone catheter by Tenckhoff and Schecter (2) in 1968 permitted secure and safe access to the peritoneal cavity. Prolonged continuous or intermittent dialysis was now possible. Second, a continuous, portable, and relatively simple dialysis technique was introduced by Popovich et al. (3) in 1976, called continuous ambulatory peritoneal dialysis (CAPD). Modification and simplification of this technique by Oreopoulos et al. (4) in 1978 resulted in fewer interruptions, increased portability, and reduced costs, leading to its popularity and acceptance.
However, peritonitis and, less commonly, catheter exitsite or tunnel infections initially led to cautious growth of this new form of chronic dialytic therapy. Rates of peritonitis as high as two to five episodes per patient year were reported in the past (5, 6 and 7). Better patient selection, improved education, and important changes in delivery systems and connectors designed to prevent touch contamination during bag exchanges have significantly reduced rates of peritonitis (8, 9 and 10). However, the major limitation of chronic peritoneal dialysis is peritonitis and its sequelae. Peritonitis is the most common reason for hospitalization (11) and for discontinuation of this form of dialysis (12). Fortunately, hospitalization rates have declined for peritoneal dialysis patients secondary to decreased peritonitis rates and use of intraperitoneal (as opposed to intravenous) antimicrobics when needed.
Infections in patients on peritoneal dialysis are largely preventable. Knowledge of the epidemiology and pathogenesis of these infections and sources of infecting microbes is essential to design effective prevention and control strategies.
METHODS OF PERITONEAL DIALYSIS
Peritoneal dialysis may be performed in various settings and with a number of techniques. It involves infusing a dialysis solution composed of balanced salts and various concentrations of glucose into the peritoneal cavity by means of a catheter and achieving ultrafiltration by hyperosmolality; retained metabolites traverse the peritoneum from the bloodstream to the solution.
Acute Peritoneal Dialysis
Acute peritoneal dialysis is generally limited to the patient with newly diagnosed acute renal failure or to other circumstances in which dialysis is anticipated for only a few days. It has now largely been replaced by continuous renal replacement therapy. Its origins date back to the 1920s (8). A rigid catheter is inserted into the peritoneal cavity at the bedside after making a small incision, and manual exchanges are performed every 1 to 3 hours as necessary (13). The procedure confers a significant risk of complications, including bowel perforation. Infection is common, especially in cannulations persisting for more than a few days. Some reasons include same location of entry and exit site, lack of an implanted cuff barrier to bacterial migration, migration of the catheter with resultant serosal injury, and the need for frequent exchanges; each poses a risk of contamination.
Chronic Peritoneal Dialysis
Patients with chronic renal failure require maintenance peritoneal dialysis to alleviate symptoms of uremia and correct other metabolic abnormalities. Chronic peritoneal dialysis did not become an acceptable therapeutic alternative to hemodialysis until the mid-1960s, when a semipermanent implantable silastic catheter was developed by Palmer et al. (14) and modified by Tenckhoff et al. (2,5). The Tenckhoff catheter is still the most frequently used peritoneal dialysis catheter today (15). Repeated insertions of a peritoneal catheter were no longer necessary to deliver dialysate. The catheter, composed of pliable silicone and usually containing two extraperitoneal Dacron cuffs, is inserted through one incision and tunneled through a subcutaneous tract until the outer end emerges from a new exit site. The Dacron cuffs initiate an inflammatory response
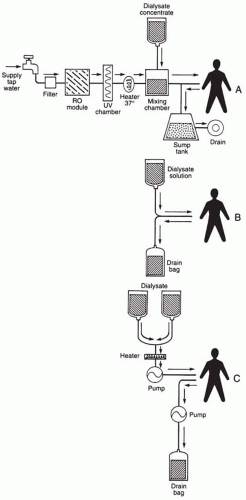
in the subcutaneous tissue near the exit site and deep in the abdominal wall, helping to seal the catheter in place, prevent fluid leaks, and limit bacterial migration around the catheter. Chronic peritoneal dialysis can be performed either intermittently or, as is common today, continuously (Fig. 64-1).
in the subcutaneous tissue near the exit site and deep in the abdominal wall, helping to seal the catheter in place, prevent fluid leaks, and limit bacterial migration around the catheter. Chronic peritoneal dialysis can be performed either intermittently or, as is common today, continuously (Fig. 64-1).
Chronic Intermittent Peritoneal Dialysis
Chronic intermittent peritoneal dialysis (CIPD) uses prolonged periods in which continuous dialysis is performed, thus permitting at least 48 hours of freedom from dialysis. To perform dialysis, a closed automated dialysis system is used to deliver dialysate to the patient (5, 6 and 7). To simplify the process and to reduce costs, an automated peritoneal dialysis system was developed that used reverse osmosis (RO) capable of producing sterile pyrogen-free water from tap water, which is then mixed with dialysis concentrate (7). These machines gained widespread popularity in the mid-1970s. Although RO proved to be effective in removing bacterial counts by as much as four logs (16), additional backup systems using heat or ultraviolet (UV) irradiation were added to ensure sterile water. Rates of peritonitis were reduced with these closed systems; however, the machines were found to be quite demanding in terms of maintenance, care, monitoring, and disinfection and may themselves provide a reservoir for pathogens (17). With the meteoric rise of simpler procedures (described later), these machines have largely been replaced.
Continuous Ambulatory Peritoneal Dialysis
CAPD is a form of closed-system continuous dialysis that is machine free. Patients on CAPD manually exchange dialysate, usually four times daily, by using dialysate delivered by gravity into the peritoneal cavity. Empty bags, connected to the catheter by extension tubing, collect the effluent, also by gravity, at the end of the dwell time. Fluid from the last exchange of the day dwells overnight in the peritoneal cavity. The technique was pioneered by Popovich et al. (3) in 1976 but initially suffered from high rates of peritonitis and patient inconveniences because of bottled dialysate. Oreopoulos et al. (4) modified the process and replaced the bottled dialysate with plastic dialysate bags, improving convenience, reducing manipulations, and lowering rates of infectious complications. CAPD is, thus, performed without the necessity of machines, is portable, and is simple to learn and perform (10,18, 19, 20 and 21).
Continuous Cycling Peritoneal Dialysis
A variant of CAPD, continuous cycling peritoneal dialysis (CCPD) combines the principles of continuous automated dialysis during the night with those of prolonged dwell time dialysis during the day by use of a machine cycler allowing for frequent exchanges (22). CCPD has many advantages, including eliminating active dialysis during the day, reducing the number of exchanges, and possibly reducing rates of peritonitis. However, CCPD may be associated with a faster rate of first-episode peritonitis than CAPD (23). Other disadvantages include cost, machine dependency, and lack of portability. Simpler cyclers will make CCPD an increasingly popular technique. CCPD became more prevalent than CAPD in 2001 and, as of 2007, is approaching twice the prevalence of CAPD (1).
DEFINITIONS
There are a number of infections associated with peritoneal dialysis. By definition, peritonitis signifies inflammation of the peritoneal membranes as a result of infection or other insult. For clinical purposes, the definition proposed by Vas (22), consisting of any two of the following three criteria, is often used to establish a diagnosis of peritonitis: cloudy peritoneal effluent containing more than 100 neutrophils/mm3, abdominal pain or tenderness, and microorganisms in the peritoneal fluid.
Exit-site infections are usually characterized by the presence of pain, erythema, tenderness, or induration of the
catheter site often accompanied by purulent discharge. Infection, when present, is commonly limited to the area between the cutaneous surface (exit site) and the superficial Dacron cuff embedded in the subcutaneous tissue near the skin.
catheter site often accompanied by purulent discharge. Infection, when present, is commonly limited to the area between the cutaneous surface (exit site) and the superficial Dacron cuff embedded in the subcutaneous tissue near the skin.
With tunnel infections, in which the area between the two Dacron cuffs is commonly referred to as the tunnel (the other cuff is embedded deep in the abdominal wall near the peritoneum), signs of infection include induration, tenderness, or redness of the overlying tissues with or without overt abscess formation.
EPIDEMIOLOGY AND RISK FACTORS ASSOCIATED WITH INFECTION
Regardless of the method of dialysis, infection, especially peritonitis, remains a serious threat to the patient. The incidence of peritonitis associated with an acute dialysis is high, approaching 0.5% to 4% (24). The incidence of peritonitis in patients receiving chronic dialytic therapy varies from center to center and depends on the method of chronic dialysis. However, no study has actually randomized patients with chronic renal failure to receive treatment by the three different methods of chronic dialytic therapy.
Over the years, the incidence of peritonitis associated with CAPD has continued to decrease from early observations of six episodes per patient year reported in the late 1970s (25,26) to 0.35 episodes per patient year documented by 2004 (27). The initially precipitous drop in infections was largely attributable to enhanced center experience and training (26), substituting plastic dialysis bags for glass bottles, reducing the number of connect-disconnect times (4), incorporating titanium connectors to connect tubing to catheters (28), and developing other methods to reduce touch contamination during bag exchanges (28, 29 and 30). Currently, the peritonitis rate is down to an average of one infection per 25 patient months of dialysis (31).
Clearly, the risk of developing peritonitis on CAPD increases with time. The period of greatest risk is the first few months of therapy. By the end of 6 months of treatment, the probability of developing at least one episode of peritonitis is at least 30% (32). This risk increases to 50% by the end of 1 year of treatment, 70% by 2 years, and approaches 80% by 3 years of uninterrupted therapy (26). More than half of all episodes of peritonitis occur in only 25% of all patients on CAPD. Twenty percent of patients develop three or more infections each year, whereas others remain free of infection for 3 or more years (10,33,34).
Several factors place a patient at increased risk for infection, especially peritonitis. These factors have been best studied in patients receiving CAPD. Although age or gender (35) does not appear to be important risk factors [rates may be higher in young children who perform their own therapy as opposed to children who obtain assistance from another family member (36)], underlying disease states may be important. For example, diabetic patients have been found to have higher rates of both peritonitis and exit-site infections (37). Lack of compliance with asepsis, lapses in technique, low patient motivation, depression, lack of social support, fewer years of education, and lower socioeconomic status all have been found to be contributing factors to infection (38). Both African Americans (39) and Native Americans (40) are at increased risk. The type of catheter design and operator do not appreciably influence rates of peritonitis (41). Antibiotic prophylaxis at time of catheter placement may decrease infection risk (42,43). Vancomycin use in this setting appears superior to cephalosporin use in prevention of early peritonitis (42). However, routine use of vancomycin is discouraged for fear of VRE development (15). Downward direction of the exit site also lowers rates of peritonitis (44). Data have demonstrated that catheters containing both a superficial and a deep Dacron cuff (double-cuffed catheters) were associated with significantly lower rates of peritonitis than single-cuffed catheters (41) and may be associated with longer catheter survival and fewer exit-site complications (45). However, another study found that single-cuffed catheters were not inferior if the single cuff was placed in the deep position (46). Studies have also confirmed that both the type and the method of connection used between the dialysis bag and the indwelling peritoneal catheter can influence rates of peritonitis. Patients using connection devices permitting flush before fill systems such as Y-sets (30) or using disconnect systems that sterilize the connection, such as UV radiation (47), had rates of peritonitis significantly lower than those of patients using standard spike connectors (29). Manual spiking of dialysis bags is a discouraged procedure (48). Finally, patients who were prescribed intraperitoneal medications and added these medications themselves had higher rates of peritonitis (29).
Intermittent peritoneal dialysis appears to result in lower rates of peritonitis when compared with CAPD. Perhaps much of this reduction can be attributed to the need for less frequent manipulations. In fact, patients on CIPD perform only 156 to 208 connect-disconnect procedures per year, as opposed to the more than 1,400 required for CAPD. Likewise, patients on CCPD appear to become infected at rates between those described for CIPD and for CAPD (18); these patients perform approximately 700 connect-disconnect procedures annually.
Exit-site or tunnel infections occur more commonly in individuals with concomitant peritonitis. Studies have also demonstrated that nasal carriers of Staphylococcus aureus are at increased risk for infection (49, 50, 51, 52, 53 and 54). Persistent carriage of S. aureus at the catheter exit site or the anterior nares is associated with a threefold increase of CAPD infections than compared with intermittent carriers (55). Another study found that frequent and comprehensive washing (ablution) combined with intranasal mupirocin significantly decreased S. aureus carriage and CAPD-related S. aureus peritonitis (56). Diabetics may also be at increased risk for infection, although this observation may be confounded by the observation of high carriage rates of S. aureus in these patients (57). The overall risk of an exit-site or tunnel infection in a patient receiving CAPD approaches 0.2 to 0.7 episodes per patient year (51,58). Half of patients on CAPD do not develop exit-site infections within 2 years of catheter placement.
Epidemics of peritonitis in patients receiving chronic dialysis have been observed, especially in patients receiving CIPD via machines that use RO to sterilize water that then mixes with a dialysate concentrate (17,59,60). Outbreaks
have also occurred as a result of delivering contaminated dialysate to the patient, either directly (61,62) or indirectly by use of water baths to heat the dialysate before infusion (63, 64 and 65). Contaminated disinfectants used to clean exit sites and tubing ports have also resulted in outbreaks of infection (66).
have also occurred as a result of delivering contaminated dialysate to the patient, either directly (61,62) or indirectly by use of water baths to heat the dialysate before infusion (63, 64 and 65). Contaminated disinfectants used to clean exit sites and tubing ports have also resulted in outbreaks of infection (66).
PATHOGENESIS
Routes of Infection
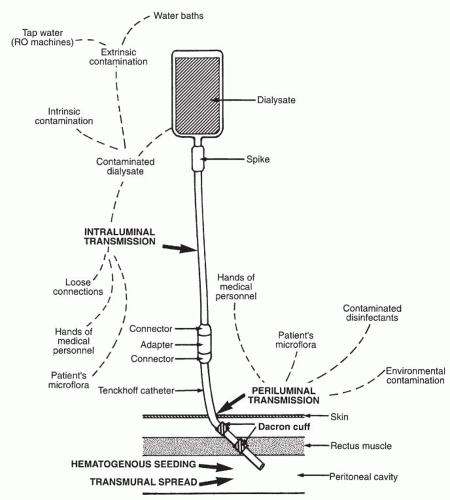
The four major pathways resulting in peritonitis in patients on dialysis are schematically represented in Figure 64-2. These include intraluminal transmission of microorganisms (microorganisms gaining entry through the infusion system); periluminal infections (infection of the catheter site with resultant local infection and, at times, spread into the peritoneum); transmural infections (peritonitis as a result of intestinal injury, perforation, or transmigration of microorganisms); and hematogenous spread, usually from a site of infection elsewhere. Exit-site or tunnel infections almost always result from a periluminal infection, although peritonitis can cause infection at the deep Dacron cuff of the silastic catheter with resultant tunnel infection or abscess formation.
Few studies have examined the most common means by which peritonitis develops in patients receiving acute dialytic therapy. Clearly, infection of the catheter site with resultant spread into the peritoneum is a major route of infection. Unlike the situation with chronic dialysis, the cannula is usually inserted directly into the peritoneum after a stab wound is made. A protective tunnel with stabilization by Dacron cuffs is not usually made for short-term acute dialysis. Another important means by which peritonitis may develop in these patients is inadvertent perforation of the bowel during blind catheter placement or as a result of perforation from migration of the rigid catheter during dialysis with injury to the bowel wall. Microorganisms can also be introduced in the lumen during bag or tubing changes.
Contrast this scenario with what is observed in patients on chronic forms of peritoneal dialysis. It appears from inferential and intervention studies that the most important route of infection in these patients is intraluminal. Intraluminal contamination can occur during the numerous connect-disconnect manipulations by means of loose-fitting connectors or malfunctioning clamps, through defects in the plastic tubing or bags, or from the dialysis fluid itself. First, peritonitis occurs at least twice as often as exit-site infections in patients on chronic dialysis (27), suggesting that microorganisms are instilled into the peritoneal cavity. Second, the most common microorganisms causing peritonitis are coagulase-negative staphylococci rather than S. aureus, a microorganism found more frequently as a cause of periluminal infections (67). Third, studies have found that a major cause of peritonitis in patients on chronic dialysis is poor technique or observed breaks in technique resulting in intraluminal contamination (68). Fourth, CIPD or CCPD, methods associated with fewer manipulations, have consistently been associated with fewer infections (18). Finally, incorporating devices or procedures to reduce touch contamination have resulted in fewer infections (29,30).
Contaminated Dialysate
Intrinsic contamination of dialysate has been reported infrequently and may result in infective peritonitis (61) or a sterile peritonitis resulting from delivery of endotoxin (62). In-use or extrinsic contamination may occur during bag exchanges. Fortunately, commercially available dialysate does not support the growth of staphylococci, the most common pathogen responsible for infection, although some gram-negative microorganisms proliferate readily if introduced (69,70). Water-adapted microorganisms such as Mycobacterium chelonae-like microorganisms and Pseudomonas species have caused outbreaks of peritonitis in patients on CIPD (17,60). Microorganisms such as M. chelonae-like organisms not only can live in chlorinated water but also may survive exposure to high concentrations of disinfectants such as formaldehyde (71).
Infection of the catheter site is the second most common cause of peritonitis and the leading cause of exit-site infections in patients on chronic peritoneal dialysis. The implanted catheter never forms a complete sealed junction with the skin; thus, microorganisms are present within the exit site and can result in infection. Although the superficial embedded Dacron cuff is a reasonable barrier, limiting the migration of microorganisms deeper into the abdominal wall or to the peritoneum, its efficacy is clearly not 100% (72). Up to 17% of patients who develop an exit-site infection also have peritonitis (67). S. aureus carriers are at high risk for developing an exit-site infection (50, 51, 52, 53 and 54,57) as are diabetics (73).
Transmural infections occur as a result of abdominal perforation or injury, inflammation of the serosal surfaces,
or transmural migration (74). Rates of peritonitis resulting from intestinal microorganisms are higher in patients with preexisting diverticular disease (75). Infection of the peritoneum or exit site by the hematogenous route is uncommon.
or transmural migration (74). Rates of peritonitis resulting from intestinal microorganisms are higher in patients with preexisting diverticular disease (75). Infection of the peritoneum or exit site by the hematogenous route is uncommon.
Host Defense Mechanisms
For peritonitis to develop, the patient’s host defenses must not be able to contain, destroy, and remove the invading pathogens. Peritoneal fluid normally contains up to 200 cells/mm3, of which more than 80% are mononuclear cells predominately macrophages (76, 77 and 78). These cells represent the primary cellular barrier against infection (79); patients prone to infection may have fewer macrophages available to combat infection (80). Many microorganisms causing peritonitis require opsonization by heat-stable substances, such as immunoglobulin G (IgG) and other specific antibodies, or heat-labile components, including complement for efficient removal. Deficiency in IgG or C3 may also predispose patients to infection, as would neutropenia (81).
It is well known that the delivery of dialysate into the peritoneal cavity has a direct adverse effect on host defense mechanisms because of the effects of the low pH and hyperosmolarity of the dialysate. Both acidity and hyperosmolarity reduce the ability of macrophages and polymorphonuclear leukocytes to phagocytize and kill microorganisms (82, 83 and 84). Also, the presence of extra liters of fluid in the peritoneal cavity during dialysis results in a marked dilutional effect on both cellular and humoral protective factors, resulting in fewer leukocytes per milliliter and a relative opsonic deficiency (81,85).
Obviously, an indwelling peritoneal catheter has adverse effects on the host. A conduit between the outside environment and the peritoneum now exists. The catheter may act as a foreign body, initiating inflammatory changes that predispose to infection, and can serve as a substrate on which colonization may be established. Although silastic catheters appear to be less thrombogenic than polyurethane catheters (86), all catheters eventually become coated with a fibrin sheath (87). Microorganisms can become embedded in this sheath or in the biofilm produced by many microorganisms, resulting in proliferation with resultant infection. This protective environment may be responsible for the difficulty in eradicating infection by seemingly appropriate antibiotics or for relapses of infection (88).
ETIOLOGIC AGENTS
Causative Microorganisms of Peritonitis
Although numerous microorganisms have been isolated from infected patients on peritoneal dialysis, most of these microorganisms are skin commensals such as coagulasenegative staphylococci (Table 64-1) (22,33,34,51,67,73,92). At least two thirds of all episodes are caused by grampositive microorganisms; Staphylococcus epidermidis is isolated most frequently. The second most common microorganism is S. aureus, followed by various streptococcal species. Gram-negative bacteria account for 20% to 30% of all episodes, with E. coli and other members of the family Enterobacteriaceae most prevalent. Fewer than 10% of episodes of peritonitis are due to Pseudomonas aeruginosa or related microorganisms. Anaerobes are uncommon. When anaerobes are present, the possibility of peritonitis from bowel perforation increases, especially if polymicrobic peritonitis is found.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree