Infection Risks of Endoscopy
John Holton
Endoscopic procedures are used worldwide for both diagnostic and therapeutic interventions. Considering the numbers of endoscopies that must be performed annually, the incidence of infection is comparatively low and estimated at one per 1.8 million examinations (1,2,3), although increasing concern has been expressed at cross-contamination during the decontamination process (4). Endoscopic procedures are becoming increasingly complex, particularly in the field of keyhole surgery; however, percutaneous endoscopic surgical procedures have impacted beneficially on the postoperative wound infection rate and by reducing hospital stay have also had an economic benefit. It is therefore important that the potential cross-infectious hazards from the instruments are reduced to a minimum by correct decontamination procedures particularly as endoscopes are the commonest medical device associated with outbreaks of infection (5,6,7).
Many endoscopic procedures are carried out with allmetal instruments and they are thus comparatively easy to decontaminate by autoclaving. There are still, however, large numbers of endoscopies performed with instruments that are flexible and heat sensitive. It is this group of instruments that presents a considerable challenge to effective decontamination, in part because of their complex internal structure, with several very narrow bore channels and difficult to clean valves and valve seats. An additional factor to be considered is the heavy workload on a clinic and consequently a short turnaround time between patients, thereby potentially making effective decontamination problematic.
Currently, there are circumstances that set a particular challenge to the safe decontamination, not only of flexible but also rigid endoscopes, and these circumstances relate to viral and prion contamination of instruments (8). These circumstances take the emphasis for the safe reuse of instruments away from simply killing adherent microorganisms to removal of contaminating “soil” that may contain microbial nucleic acids, hazardous protein, and endotoxin. The availability of the polymerase chain reaction (PCR) has demonstrated that following decontamination procedures (9) it is possible to still detect the presence of microbial nucleic acid and that, although it may not be an infective hazard, it may well be hazardous for the patient by other mechanisms. These considerations and the concern regarding biofilms have led to a reevaluation of current decontamination procedures by professional organizations and to the circulation of new protocols to deal with contaminated endoscopes (10,11,12,13,14, 15, 16 and 17,18,19,20,21,22,23).
To standardize the decontamination procedure for endoscopes, the instruments are now almost universally processed in automated washer/disinfectors. This does not obviate the need for an initial manual cleaning, which is vital to the whole decontamination process, but does ensure that all endoscopes are decontaminated in an identical fashion and frees the endoscopy nurses for other duties in the clinic. There is, however, a downside to the use of automated washer/disinfectors, which relates to recontamination of endoscopes by the machine after the disinfection stage. This is due to the growth of microorganisms within a biofilm present in the tanks and pipes of the washer/disinfector and thus recontamination during the final rinse prior to removal of the instrument from the machine (24). This problem has led to the reporting of pseudo-outbreaks of tuberculosis following bronchoscopy and to actual infection of patients with environmental gramnegative microorganisms. The manufacturers of automated washer/disinfectors have had to redesign the internal architecture of the machines and to otherwise modify them by including a self-disinfection cycle. The problem has also led to the development of systems for the provision of sterile water to the machine from the potable water supplies.
Aldehydes, for example, 2% glutaraldehyde, are still probably the commonest disinfectants used worldwide for flexible endoscopes (25, 26, 27, 28 and 29) although in some countries, including the United Kingdom, they have been withdrawn from the market and this has of necessity led to the use of other disinfectants. Glutaraldehyde does have two major disadvantages despite its efficacy as a disinfectant. It is now recognized to be a major cause of occupational allergy, giving rise to both pulmonary and skin hypersensitivity (30,31). Its other main disadvantage is that it acts as a fixative, and with concern expressed over prion proteins and the emphasis placed on soil removal from endoscopes, this characteristic in a disinfectant is unwelcome. The disinfectants that have replaced glutaraldehyde (in the United Kingdom) are very effective in killing microorganisms but are far more corrosive both to the endoscope and to the washer/disinfector.
The area of endoscope decontamination is thus currently in a state of flux, and further developments are anticipated with respect to the processes of decontamination, the nature of disinfectants, and the materials from which endoscopes are manufactured.
TYPES OF ENDOSCOPES
Endoscopes are constructed from a diverse range of materials including plastic, metal, glass, and adhesives. They generally have a complex internal construction with narrow bore channels, external ports, and valves. Many different endoscopes are now produced for a variety of medical interventions, both therapeutic and diagnostic, including bronchoscopy, arthroscopy, laparoscopy, colonoscopy, gastroscopy, and cystoscopy. The endoscopes may be flexible or rigid, the latter usually made entirely of metal and are thus relatively easily decontaminated compared to the flexible endoscopes. They may be used as direct viewing instruments or for the collection of biopsy specimens, as video endoscopes, or as endoscopes with an ultrasound attachment used for diagnostic purposes.
Endoscopes can be classified as critical instruments— those that penetrate the skin or sterile body cavities—or as semicritical instruments—those that are in contact with mucous membranes. However, this distinction is not clear-cut, as many semicritical instruments may be in contact with pathologic lesions, where the local defenses are breached, or they are used to take specimens, thus breaching local defense mechanisms.
Critical Instruments
These instruments include laparoscopes, vascular and neurological endoscopy, cystoscopes, and arthroscopes. Some of these instruments such as the cystoscopes and laparoscopes may be rigid in construction, made out of metal, and are thus autoclavable.
Laparoscopes are used for visualizing the peritoneal cavity, penetrate the skin, and are increasingly used as surgical equipment involved in intraperitoneal operations such as cholecystectomy, hysterectomy, hernia repair, and tubal ligation. Similar instruments may also be used in the thoracic cavity for some surgical procedures, in cosmetic surgery for rhytidectomy, and in general surgery for thyroidectomy. Angioscopy is used for atherectomy, embolectomy, and direct inspection of vessels. Endoscopes are used in neurology for III ventricle ventriculoscopy, in cases of hemorrhage-related obstructive hydrocephalus, and for transsphenoidal resection of pituitary adenoma. Arthroscopes are also rigid and autoclavable and are used for inspecting joint spaces and surgical procedures including meniscectomy. These instruments are also used percutaneously. Hysteroscopes are used for visualizing the uterus, for removing polyps, for biopsies, and for resection of submucous fibroids. Cystoscopes are often rigid, although ureteroscopes are flexible. These instruments are used for visualizing the urinary tract, taking biopsies, removing small tumors and calculi, and dilating stenosed regions of the urinary tract. They may be passed into the renal tract through the urethra or directly into the renal pelvis percutaneously.
In general, the flexible operative endoscopes are heat labile and should be sterilized by ethylene oxide or gas plasma. The rigid ones may be autoclaved.
Semicritical Instruments
These instruments include gastroscopes, duodenoscopes, sigmoidoscopes, proctoscopes, colonoscopes, bronchoscopes, and laryngoscopes. Gastroscopes, duodenoscopes, and colonoscopes are long, flexible instruments, usually with four channels (suction, biopsy, air, and water) and corresponding ports and valves. The suction and biopsy channels are often combined within the insertion tube. Their intricate construction makes them difficult to clean, and the materials from which they are made make them difficult to decontaminate. These instruments are inserted through one of the natural orifices of the body, which has a rich normal flora. Bronchoscopes are thus categorized as semicritical despite the fact they enter a sterile body cavity. These instruments are used both diagnostically and for minor surgical procedures such as polyp removal or diathermy.
Accessories
A wide range of accessories is available for both critical and semicritical endoscopes including forceps, snares, diathermy, bougies, sphincterotomy knives, and lasers. Many of these accessories can be autoclaved, but increasingly manufacturers are supplying single-use disposable accessories. Laser and ultrasonic probes are expensive and not able to be autoclaved.
ETIOLOGY
The commonest microorganisms that cause endoscopyassociated infections or pseudoinfections are opportunistic gram-negative bacteria and mycobacteria that are associated with moisture or biofilms on an endoscopy processing apparatus (32,33). Microorganisms that have frequently been isolated include Pseudomonas species (34), Serratia marcescens, Klebsiella, Escherichia, and Salmonella species (35, 36, 37, 38, 39, 40, 41 and 42). Salmonella sp. is easily diagnosed as cross-infection due to a poorly decontaminated endoscope because this microorganism would not normally be found in the environment of an endoscopy room as a contaminant. Cross-contamination with Salmonella would be likely to cause an infection, even in relatively healthy individuals, in comparison to the environmental opportunist microorganisms commonly linked to failed endoscope decontamination, such as Klebsiella and Pseudomonas. Most of the cases of transmission of Salmonella, and there have been relatively few, date from the 1970s to 1980s, and in all cases, disinfectants were used that would be regarded as inappropriate by current standards. The agents that were used to decontaminate the endoscopes were skin antiseptics—chlorhexidine, cetrimide, povidone-iodine, hexachlorophene, and quaternary ammonium compounds. The majority of reported infections occurred prior to 1983, with only three more cases reported by 1992 and none to the current time. Since the late 1980s, glutaraldehyde and more recently other agents have been used to decontaminate endoscopes, with the effect that there are fewer reported incidents of crossinfection from an endoscope contaminated with enteric gram-negative bacteria.
Bronchoscopy has been associated with contamination or infection caused by Mycobacterium tuberculosis, M. kansasii, M. chelonae, and M. abscessus (24,43, 44, 45, 46 and 47). Cystoscopy has been associated with infections by Escherichia, Enterococcus, and Proteus species (48). Percutaneous
endoscopy has been associated with skin flora and Staphylococcus aureus surgical site infections (49).
endoscopy has been associated with skin flora and Staphylococcus aureus surgical site infections (49).
There is little evidence of viral transmission after endoscopy. Both bronchoscopes and gastroscopes become contaminated with human immunodeficiency virus (HIV) when used on patients with the acquired immune deficiency syndrome (AIDS), yet there is no evidence of transmission following endoscopy. Studies have shown that mechanical cleaning of endoscopes removes even high concentrations of HIV and that glutaraldehyde rapidly inactivates the virus (50). There is a single well-documented case of hepatitis B virus (HBV) transmission between patients (51), but most studies have not been able to document transmission. Of 394 patients followed up after exposure, none showed clinical evidence of infection (52). There have been three reported cases of hepatitis C virus (HCV) transmission, one following endoscopic retrograde cholangiopancreatography (ERCP), and two following colonoscopy, and in all cases decontamination was found to have been ineffectively carried out (53). In a study of 19 patients with HCV using molecular techniques to detect the virus, a blood sample taken from the patient was positive prior to the procedure, and 53% of the endoscopes were contaminated with the virus immediately after removal, but after both mechanical cleaning and mechanical cleaning followed by immersion in a disinfection, none were contaminated (54). Thus, current decontamination procedures appear to be sufficiently robust to prevent viral transmission following endoscopy.
Less frequently identified pathogens that may be transmitted by endoscopic procedures include Helicobacter pylori (55,56), Shewanella spp. (57), Trichosporon asahii (58), and Strongyloides (59). Other microorganisms that may be transmitted by endoscopy include Clostridium difficile, Cryptosporidia, and enteroviruses.
PATHOGENESIS
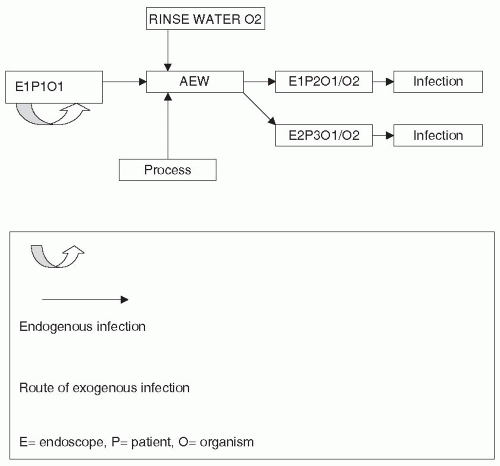
Infections are derived either from an external source (exogenous) or from the patient’s own microflora (endogenous). (Fig. 62-1) Endoscopically transmitted infection reported in the literature has been mainly exogenous, from inadequately decontaminated endoscopes, although endogenous infections have also been reported, particularly in association with urologic or percutaneous procedures.
Exogenous Spread of Infection
There are two main reasons for microorganisms being transmitted to a patient from an endoscope, which are to some extent related. On the one hand, the endoscope may be inadequately decontaminated. On the other hand, microorganisms produce and reside in a biofilm when in a moist environment, such as an endoscope or an endoscope washer/disinfector (AEW). Many bacteria secrete a carbohydrate substance, frequently called “slime,” which forms the glycocalyx or matrix (the biofilm) within which the microorganisms can survive (60,61). Often, biofilms contain complex microbial communities. The dynamics of the biofilm are still poorly understood, but what is certain is that microorganisms within the biofilm are more resistant to biocides than adherent but non-biofilm-associated bacteria or planktonic bacteria (62,63). Additionally, biofilms and the associated bacteria are resistant to hydrodynamic shear forces. Both these characteristics make eradication of microorganisms from endoscopes or endoscope washer/disinfectors difficult and predispose to failure of decontamination processes. The net result is that microorganisms are still present on the endoscope, or the endoscope becomes recontaminated following the decontamination procedure (64,65) by poor quality rinse water. Thus, in Fig 62-1, the endoscope (E1) will become contaminated
during the procedure with microorganisms (O1) from the first patient (P1). The endoscope will then be decontaminated in an Automated Endoscope Washer/Disinfector (AEW) and if the procedure is inappropriate or poorly carried out the microorganism will not be removed or killed and when the same endoscope is used on another patient (E1P2O1) an infection may occur. The AEW may also act as a source of contamination of endoscopes. Microbes derived from patients (O1) or from contaminated rinse water (O2) may reside in the biofilm and contaminate endoscopes processed in the machine thereby infecting other patients (E2P3O1/2).
during the procedure with microorganisms (O1) from the first patient (P1). The endoscope will then be decontaminated in an Automated Endoscope Washer/Disinfector (AEW) and if the procedure is inappropriate or poorly carried out the microorganism will not be removed or killed and when the same endoscope is used on another patient (E1P2O1) an infection may occur. The AEW may also act as a source of contamination of endoscopes. Microbes derived from patients (O1) or from contaminated rinse water (O2) may reside in the biofilm and contaminate endoscopes processed in the machine thereby infecting other patients (E2P3O1/2).
Airborne infection from staff members in the endoscopy room during percutaneous surgical procedures, although possible, is unlikely owing to the small incision produced. During endoscopic surgery, the video screen may act as a source of contamination of the surgeon’s hands, as the electrostatic field generated by the screen facilitates the transfer of microorganisms from the screen to the gloved hands of the surgeon (66).
On the other hand, spread of infection from the patient to the staff is a very real risk during bronchoscopy, particularly when dealing with patients who have tuberculosis. Similar concern has been expressed about staff members acquiring infection with HIV when performing endoscopy on patients who are infected (67). In one study of 427 urologic procedures, contamination of the surgeon’s skin or mucous membranes occurred in 32%. Thirty-three percent were endoscopic procedures and in these, contamination of the face or eyes occurred in 46% (68). Although in practice the risks of infection are small, the risks of contamination in some procedures are high, and appropriate physical precautions have been almost universally introduced.
In addition to the transfer of microorganisms to the patient, who may then become colonized, whether or not the patients develop an infection is due to other contributory factors such as their underlying medical condition, the treatment they may be receiving, and whether they already have a focus of infection such as an obstructed bile duct (69).
Endogenous Spread of Infection
Endogenous infection may be due to transfer of microorganisms from one site to another during the insertion or removal of the endoscope. Mouth flora may be transferred to the stomach or to the bronchi during gastroscopy or bronchoscopy. Mouth, stomach, or duodenal flora may be transferred to the biliary or pancreatic system during ERCP. Intestinal flora may contaminate the oral cavity on removal of a gastroduodenoscope or ERCP. Similarly, skin flora may be introduced into the peritoneal cavity, pleural cavity, or joint; vaginal flora may be introduced into the uterus; and fecal, skin, or urethral flora may be introduced into the bladder or kidneys during urological procedures.
INFECTIONS ASSOCIATED WITH ENDOSCOPY
The insertion, manipulation, or removal of the endoscope may be associated with bacteremia, usually with the patient’s own microflora but rarely from microorganisms contaminating the endoscope. More usually, pseudoinfections have been reported in the literature, due to contamination of a patient’s specimen by a microorganism derived from the endoscope. Percutaneous procedures may be followed by surgical site infections or joint infections, peritonitis, bacteremia, or empyema.
Between 1974 and 2004, there were 140 outbreaks related to endoscopy reported in the world literature (70). Forty-nine percent were from the United States and 51% from 19 countries other than the United States. Bronchoscopy accounted for 47% of infections and 94% of pseudoinfections in the United States and 21% of infections and 76% of pseudoinfections outside the United States. Gastrointestinal endoscopy accounted for a similar percentage of infections, but only 6% of pseudoinfections within the United States and 76% of infections and 24% of pseudoinfections outside the United States. Overall, bacteria were the principal cause of infections and pseudoinfections.
The commonest cause of toxic reactions was glutaraldehyde, which has now been withdrawn from use in the United Kingdom. The most frequent cause of outbreaks related to inadequate decontamination practices followed by contamination of the AEW in the United States and the use of contaminated water outside the United States. Over this period of study, the primary cause of decontamination failure fell from 72% to 47% in the United States and 81% to 70% elsewhere. Since 1990, equipment malfunction has been identified as a cause of failure in the United States (8%) and elsewhere (4%) and AEW contamination accounting for 25% and 4% in the United States and elsewhere, respectively. A key outcome of this study was that by better adherence to decontamination guidelines, 90% to 97% of the outbreaks could have been prevented both within and outside the United States. During this period of study, there were 14 deaths, 1.9% of the total number of exposed patients in the United States and 6 deaths elsewhere (1% of contaminated patients). The mean length of an endoscopyrelated outbreak was 182 days in the United States and 202 days elsewhere.
These data represent an underestimate as an unknown number of endoscopy-related incidents may go unreported, and in about half of the reported cases insufficient data was recorded and particularly the denominator (number of patients undergoing endoscopy) is unknown. Since this study, there have been nine further outbreaks reported from 2005 to 2008 that occurred outside the United States (71).
Endoscope-Assisted Surgery
Laparoscopic Surgery Surveys of infective complications following minimally invasive procedures are few, and there is little evidence to show they are due to contaminated endoscopes as opposed to complications of the procedure. Surveys between 1975 and 1980 of, in one case, over 100,000 laparoscopies (72) showed an infection rate of 3% to 4%, with only seven possibly being due to nonsterile equipment. In a second survey of over 10,000 laparoscopies (73), three cases of surgical site infection were reported, none of which were thought to be due to contaminated equipment. In 1991, a prospective study
of 1,518 laparoscopic cholecystectomies showed an infection rate of 0.9% to 2.0% (74). In 1999, a retrospective survey of 1,702 laparoscopic cholecystectomies (75) showed an infection rate of 2.3%, with a surgical site infection rate of 0.4%. The commonest infective complication following this procedure is septic complications after spillage of gallstones in the peritoneal cavity (76, 77 and 78), and not due to a failure of decontamination procedures. In 2002, a Cochrane Review of laparoscopic appendectomy compared to open appendectomy covering 45 studies (79) showed that wound infection following the laparoscopic procedure was half as likely as with the open procedure, but that intraabdominal abscess was three times as likely. It was not suggested that failed decontamination procedures were the cause of any of the infective complications.
of 1,518 laparoscopic cholecystectomies showed an infection rate of 0.9% to 2.0% (74). In 1999, a retrospective survey of 1,702 laparoscopic cholecystectomies (75) showed an infection rate of 2.3%, with a surgical site infection rate of 0.4%. The commonest infective complication following this procedure is septic complications after spillage of gallstones in the peritoneal cavity (76, 77 and 78), and not due to a failure of decontamination procedures. In 2002, a Cochrane Review of laparoscopic appendectomy compared to open appendectomy covering 45 studies (79) showed that wound infection following the laparoscopic procedure was half as likely as with the open procedure, but that intraabdominal abscess was three times as likely. It was not suggested that failed decontamination procedures were the cause of any of the infective complications.
In a report from India, local skin infection with M. tuberculosis followed laparoscopic surgery in eight patients (80). The endoscopes were soaked in an open tray for 20 minutes, a technique no longer used in developed countries and that emphasizes the importance of correct modern decontamination procedures.
In a study of 801 patients treated by laparoscopic distal pancreatectomy, an infection rate of 0.6 was recorded. Fewer patients have undergone total pancreaticoduodenectomy, and of 85 so reported although the morbidity was high (34%) the rate of infection was not reported (81).
Minimally invasive surgery has so far involved percutaneous entry into the abdominal cavity to perform the operative procedure with a lower infection rate compared to conventional surgery. However, the development of natural orifice transluminal endoscopic surgery (NOTES) where flexible endoscopes are used to perform intra-abdominal and intrathoracic operations may alter that, as entry into the abdominal cavity is via the vagina (82) or stomach (83). As the endoscope transverses a heavily colonized mucosal site, the procedure may be associated with a higher postoperative infection rate as there is a potential to introduce microorganisms into a sterile cavity. However, in both series, no infections were recorded and the transvaginal approach is frequently used by gynecologists without adverse infection risks. This suggests that terminal sterilization of surgical endoscopes is not required, although a protocol has been developed to sterilize these surgical endoscopes using peracetic acid (84) Further, a single-port endoscopic procedure for cholecystectomy (SPEC) has been developed in pigs (85) as an alternative to NOTES, and as there are fewer breaks in the skin, this may even further reduce the possibility of infection.
Arthroscopy
Infections following arthroscopy are uncommon, with, in one survey of 12,505 procedures, an infection rate of 0.04% being reported (86). Postprocedure infections do occur, usually with skin flora and usually due to environmental contamination rather than poor decontamination of the arthroscope. In one study, three joint infections occurred in 155 arthroscopies (87), but following alteration of environmental factors, there were no subsequent infections in 222 procedures. In one more recent study, Candida albicans infection occurred following arthroscopy. Infectious complications can also follow other endoscopic orthopedic procedures, and in one case lumbar discitis occurred following laparoscopic sacrocolpopexy (88), although there was no indication this was due to failed decontamination. Infection has also been reported following meniscus repair performed by arthroscopy. Three patients developed a septic arthritis with Staphylococcus epidermidis, and the likely source was the cannulae. In vitro studies demonstrated these cannulae could only be sterilized by an ultrasonic bath, jet washing of the lumen, and steam sterilization. (89).
Cystoscopy
Cystoscopes were among the first endoscopes to be used, and initially inadequate disinfection was responsible for infection. Many of the cystoscopes can be autoclaved, although flexible heat-sensitive cystoscopes are also used. In the 1950s, it was shown that patients were developing infections within a few days of the procedure (90). A number of disinfectants were introduced, and since the use of 2% glutaraldehyde and antibiotic prophylaxis, the postprocedure infection rate is small. In a study of 161 cystoscopies, an infection rate of 7.5% was reported with microorganisms derived from endogenous flora, giving no suggestion that failed decontamination procedures were to blame (91). In a study of 420 patients following flexible cystoscopy, 110 patients donated a postprocedure urine specimen 3 days following the investigation, with 2.7% showing evidence of infection (92). Percutaneous urologic procedures, such as nephrostomy or insertion of a ureteral endoprosthesis, in one study had a complication rate of 7%, with 0.87% being due to urinary tract infection. Minor complications of skin inflammation occurred in 5.3%, but in no case was it thought to be due to poor decontamination procedures (93). An outbreak of Pseudomonas aeruginosa infection following cystoscopy was identified in New Mexico involving 23 patients. Most of the patients had a urinary tract infection postprocedure, but 4 also had bacteremia. A multivariate analysis indicated cystoscopy was the most likely common factor (OR: 46.5). On examination, the cystoscope was positive for the microorganism and there were several breaches of the decontamination protocol identified (94). Another study in a urology unit indicating inadequate disinfection as a cause of an outbreak demonstrated that forceps were the likely source. In this case, 10 isolates of Pseudomonas recovered from the forceps were indistinguishable by pulse-field gel electrophoresis (95).
Endoscopic Vascular Surgery
Infections are also a complication of cardiovascular cannulation, although there is no suggestion that these infections are due to failed decontamination procedures, as the cannulas are sterile, single-use items. In a retrospective study between 1991 and 1998 of 22,006 procedures, there were 25 cases of bacteremia (0.11%) with 0.24% following percutaneous transluminal coronary angioplasty, 0.06% following cardiac catheterization, and 0.08% following electrophysiologic studies. The majority of the infections were with gram-negative bacteria (96) (see also Chapter 61).
In one study, 103 patients undergoing coronary artery bypass graft (CABG) had the saphenous vein removed by minimally invasive endoscopic procedure. Eight
point seven percent of the standard operative control population (9/105) developed a wound infection, whereas only 2 out of 103 developed an infection after in the endoscopic procedure (97). A further study demonstrating the lower infection rate using minimally invasive endoscopic procedures compared open surgical repair of the abdominal aorta compared to endovascular repair. The patient with an open procedure was twice as likely to develop an infection compared to the one having endovascular repair (98).
point seven percent of the standard operative control population (9/105) developed a wound infection, whereas only 2 out of 103 developed an infection after in the endoscopic procedure (97). A further study demonstrating the lower infection rate using minimally invasive endoscopic procedures compared open surgical repair of the abdominal aorta compared to endovascular repair. The patient with an open procedure was twice as likely to develop an infection compared to the one having endovascular repair (98).
Endoscopic Neurosurgical Procedures
Third ventricular endoscopy (ETV) has been used for obstructive hydrocephalus of several etiologies including removal of tumors, Chiari malformation, aqueduct narrowing, spina bifida, and following a cerebral hemorrhage. In one study of 34 procedures for obstructive hydrocephalus following cerebral hemorrhage, spanning a 15-year period of endoscopic neurosurgery, no cases of infection were reported postprocedure (99). Although this study is small, if the general trend of lower post procedure infection rates for other types of surgery is observed, then ETV will compare well with placement of an extraventricular drain (EVD) where infection rates of 10% are common (100). Further, some studies report infection rates as high as 45% for EVD. In another study of 190 patients treated by EVT for obstructive hydrocephalus (101), there were no cases of postprocedure infection reported, and in many cases an EVD or VP shunt was not needed, thus avoiding recognized infectious complications. In a study of ETV for colloid cyst removal in 55 patients, the infection rate was 0% compared to 5 of 27 patients in the control surgical procedure (102). Finally, after endoscopic removal of subcortical tumors in 21 patients, only one postoperative infection was identified. (103). The use of endoscopic procedures in neurosurgery not only results in a lower infection rate postprocedure, but there are no reports of contaminated endoscopes linked to outbreaks.
Miscellaneous Endoscopic Surgical Procedures
In a study of 251 patients who underwent thyroidectomy using an endoscopic transcervical approach, the infection rate was 2.6% compared to 7.35% in conventional surgery (104). A 10-year prospective study of endoscopic rhytidectomy in 54 patients did not record any case of postoperative infection or of cross-infection (105).
Semicritical Endoscopes
Gastrointestinal Endoscopy Infections associated with gastrointestinal endoscopy are uncommon, and several surveys dating from the 1970s have shown a rate of <1%. In a survey of over 240,000 gastrointestinal endoscopies, only 24 infective complications were reported, including four fatal cases, two of cholangitis and two of pancreatitis (106). In a further study, 116 infective complications were reported, which included bacteremia, hepatitis B, endocarditis, aspiration pneumonia, and Creutzfeldt-Jakob disease (CJD) (25). The microorganisms isolated included enteric gram-negative bacteria such as Serratia and Salmonella, environmental bacteria such as Pseudomonas, and gram-positive bacteria such as S. aureus. In a survey in the United Kingdom of 164,000 endoscopies, the infection rate was 0.74% for ERCP, but of those infected, there was a high mortality rate of 26% (107). Percutaneous endoscopic gastrostomy (PEG) is a procedure for establishing enteral feeding (108). In one study of 166 PEG procedures, the complication rate was 16.3%, with wound infections occurring in 5.4%, including one case of necrotizing fasciitis. Esophagoscopy has been linked to the transmission of Pseudomonas with, in some cases, evidence of infection and death following septicemia (109). In a study of 760 children undergoing PEG between 1994 and 2005, there was a 4% complication rate (skin infection) postprocedure in hospital rising to 20% out of hospital (110).
Lower respiratory tract infection has also followed gastroscopy, again with Pseudomonas, which probably relates to aspiration of oral secretions associated with a contaminated endoscope (111).
Following sigmoidoscopy, a 10% prevalence of bacteremia that was detectable over a period of 15 minutes has been reported, although no obvious infective complications were noted (112). Transient bacteremia has also been reported in other studies (113, 114, 115 and 116). A false sense of security may be given if using disposable rigid sigmoidoscopes as microorganisms may contaminate the nondisposable bellows or light head. In one study of 21 sigmoidoscopies, a number of enteric bacteria were detected in these two locations (117).
Procedures involving sclerotherapy with N-butyl-2 cyanoacrylate have been shown to have a high rate of bacteremia and peritonitis, ranging from 5% to 53% and 0.5% to 3%, respectively (118), and in some cases endocarditis or abscess has occurred following endoscopy (119, 120 and 121). An alternative approach is the use of a covered needle (Clisco needle) whose tip does not become contaminated during insertion of the endoscope (122). Culture of the tip of covered needles compared to noncovered needles showed a lower contamination rate for the covered needle and by implication this may lower the rate of postprocedure bacteremia. However, endoscopic variceal ligation is replacing sclerotherapy as the method of choice to control bleeding. This procedure has a 3% to 14% risk of bacteremia, with 11/67 patients developing bacteremia and 2/67 developing peritonitis (123).
Endoscopic Retrograde Cholangiopancreatography
Infection following ERCP is more common than with other forms of gastrointestinal endoscopy, particularly when the biliary tree is obstructed. A postal survey of 10,000 endoscopies showed an infection rate of 3%. Most complications were due to pancreatitis, but cholangitis and cases of infected pancreatic pseudocyst also occurred, as did a small number of cases of aspiration pneumonia (124). Exogenous infection leading to septicemia, following the use of a contaminated endoscope for ERCP, has also been reported. The microorganism isolated from the patient’s blood, the endoscope, and the water reservoir was P. aeruginosa (125). In a survey of 690 ERCPs, fever occurred in 12 patients and 5 of these died of septicemia (126). Microorganisms isolated were Pseudomonas, Klebsiella, Proteus, and Escherichia. Several other reports have documented infections following ERCP, frequently in association with biliary stasis, and thus likely to be of endogenous origin, but also following the use of inappropriate disinfectants as mentioned previously, or more recently, incidents have been reported
following recontamination of the endoscope from the endoscope washer/disinfector. In both cases, outbreaks due to Pseudomonas have been reported (127, 128, 129 and 130). In one report, the post-ERCP infection rate in one hospital increased from 1.6% to 3.6% following the use of a new automated washer/disinfector. The microorganisms causing the bacteremia were Pseudomonas and enteric gram-negative bacteria. Seven epidemic strains causing infection were genomically related as shown by macrorestriction DNA analysis and accounted for 55% of the episodes. Effective decontamination of the washer/disinfector led to a reduction in the infection rate to preincident levels (131).
following recontamination of the endoscope from the endoscope washer/disinfector. In both cases, outbreaks due to Pseudomonas have been reported (127, 128, 129 and 130). In one report, the post-ERCP infection rate in one hospital increased from 1.6% to 3.6% following the use of a new automated washer/disinfector. The microorganisms causing the bacteremia were Pseudomonas and enteric gram-negative bacteria. Seven epidemic strains causing infection were genomically related as shown by macrorestriction DNA analysis and accounted for 55% of the episodes. Effective decontamination of the washer/disinfector led to a reduction in the infection rate to preincident levels (131).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree