FIGURE 44-1. This is an LAO fluoroscopic image of a 20-pole catheter in the right atrium. Poles 1 and 2 (distal) are located in the proximal coronary sinus and poles 19 and 20 are in the high right atrium. The catheter lies across the cavotricuspid isthmus (poles 1-10) and then along the right atrial free wall (poles 11-20). The diagram in the lower right corner depicts a right atrial free wall atriotomy with a macroreentrant circuit rotating around the scar/incision (yellow arrows).
MAPPING OF INCISIONAL, PATCH, AND SCAR- RELATED MACROREENTRANT ATRIAL FLUTTER
This case is an example of atrial reentry around an area of scar/incision, termed “incisional flutter.” As is common with these tachycardias, the patient has undergone an atriotomy several years prior to onset of the tachycardia as part of valve surgery.1 Incisional AFlr usually does not respond to antiarrhythmic medication and typically requires ablation.
In addition to understanding the anatomy based on prior operative procedure notes, there are four features that aid in mapping AFlr due to incision-related scar. These features are also equally applicable to mapping and ablating any form of macroreentrant AFlr (inclusive of isthmus-dependent AFlr, AFlr/atrial tachycardias following congenital heart surgery, and postatrial fibrillation ablation AFlr):
1. Postpacing intervals.2 The differential diagnosis of the tachycardia mechanism for this case is clockwise rotation of an isthmus-dependent flutter or an incisional flutter. To diagnose the mechanism, the response to pacing is helpful. When the tachycardia is entrained by atrial pacing, the tip of the pacing catheter is in or near the reentrant circuit if the first return beat of the tachycardia recorded from the pacing catheter, after pacing is stopped, is within 30 ms of the tachycardia cycle length: post-pacing interval (PPI) measured from the pacing catheter minus the tachycardia cycle length (TCL) ≤ 30 ms.
2. Atrial concealed entrainment.3,4 When an atrial tachycardia is entrained, there is often fusion between the wave of depolarization generated from the pacing catheter colliding with the wave of depolarization of the tachycardia. The resultant P wave is a fusion beat: a P-wave morphology that is “fused” between the two sources. Progressive fusion, meaning that the P-wave morphology is changing during the first several beats of pacing, confirms that the pacing catheter is not in the reentrant circuit. However, when pacing from within the circuit, there is no fusion, and the P-wave morphology generated from the pacing catheter is identical to the P wave generated by the tachycardia. This is called “concealed entrainment” and implies that the pacing catheter is within the circuit. Although this technique is effective for mapping reentrant ventricular arrhythmias since changes in QRS morphology are obvious, change in the P-wave morphology using surface ECG analysis is subtle. Concealed entrainment, for any mechanism of macroreentrant AFlr, is therefore determined by the absence of change in the atrial activation sequence, amplitude, and morphology of intracardiac atrial recordings when pacing from within the reentrant circuit.
3. Low amplitude/fractionated electrograms.4 An essential feature of ablating non–isthmus-dependent AFlr is to identify the zone of slow conduction that is critical to the reentrant arrhythmia. This slow zone of conduction is characterized by anisotropic conduction. Anisotropic conduction is slow conduction related to local disorganized atrial architecture, often at sites of atrial myocardium intertwined with atrial scar/fibrosis. Atrial electrograms at these sites have low amplitude and are highly fractionated. Identification of these electrograms coupled with response to pacing and atrial entrainment (listed previously), help to identify the zone of slow conduction and sites to target for ablation.
These three features of assessing a macroreentrant arrhythmia (postpacing intervals, atrial concealed entrainment, and low amplitude/fractionated electrograms) are demonstrated in Figures 44-2 to 44-6 for this case of incisional AFlr (see figure legend for further explanation).
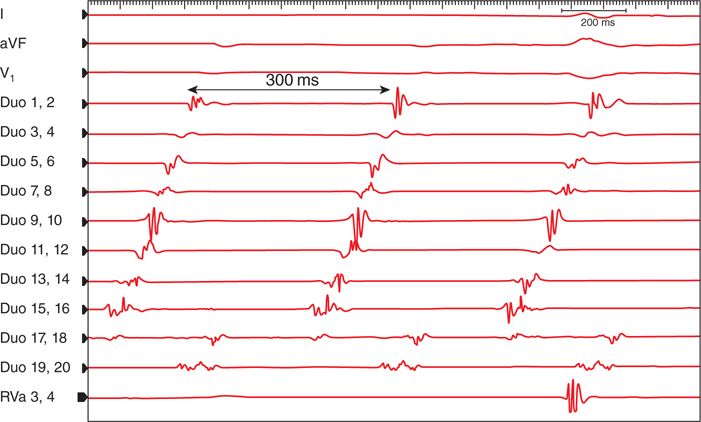
FIGURE 44-2. This is the initial intracardiac tracing of the tachycardia, cycle length 300 ms. The activation sequence is earliest at poles 17 and 18 and last at poles 1 and 2. The differential diagnosis is clockwise isthmus-dependent AFlr or incisional AFlr. The widely split electrograms on poles 17 and 18 and the late activation of poles 19 and 20 coupled with the history of a prior atriotomy suggest an incisional flutter, but response to pacing is required to confirm a diagnosis (see Figure 44-3). Abbreviations: aVF, V1, surface ECG I; 20-pole recordings labeled as Duo 1-20; RVa, right ventricular apical recording.
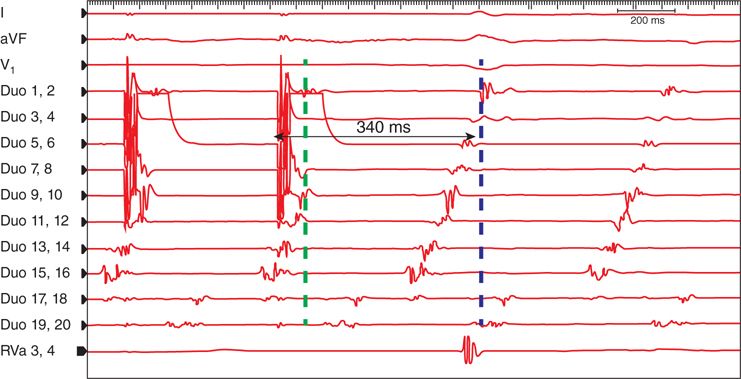
FIGURE 44-3 This demonstrates the response to pacing from poles 5 to 6. These poles are located within the cavotricuspid isthmus. If the tachycardia is an isthmus-dependent AFlr, the response to pacing should confirm this mechanism. However, pacing confirms that the mechanism is not
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree