Chapter 43 Techniques in microscopy
THE MICROSCOPE
Micrometers
Two scales are required, known, respectively, as a stage micrometer and an eyepiece micrometer. The stage micrometer is a glass slide 7.6 × 2.5 cm (3 × 1 inch) with a scale engraved on it. The scale is usually 1 or 1.1 mm long and is divided into 0.1 and 0.01 parts of a millimetre. The eyepiece micrometer may be a linear scale (Fig. 43.1A and the scale 0–10 in Fig. 43.1B) or it may be ruled in squares. The value of one eyepiece division is determined for every optical combination to be used, a note being made in each case of the objective eyepiece and length of draw-tube.
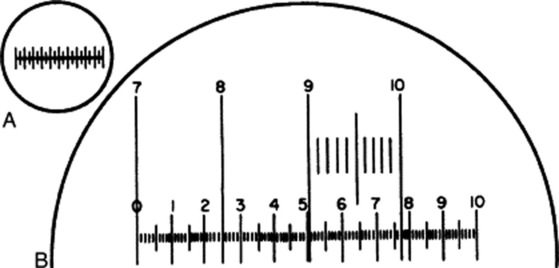
Fig. 43.1 A, Eyepiece micrometer; B, eyepiece micrometer superimposed on portion of stage micrometer scale.
To do this, unscrew the upper lens of the eyepiece, place the eyepiece micrometer on the ridge inside, and replace the lens. Put the stage micrometer on the stage and focus it in the ordinary way. The two micrometer scales now appear as in Fig. 43.1B, when the 4 mm objective is in use. In the example figured, it will be seen that when the 7 line of the stage micrometer coincides with the 0 of the eyepiece, the 10 of the stage coincides with 7.7 of the eyepiece. As the distance between 7 and 10 on the stage scale is 0.3 mm, 77 of the small eyepiece divisions equal 0.3 mm or 300 μm; therefore, 1 eyepiece division equals 300/77 or 3.9 μm.
Camera lucida
Various forms of apparatus have been designed so that a magnified image of the object under the microscope may be traced on paper. The Swift-Ives camera lucida and the Abbé drawing apparatus are examples. The former fits over the eyepiece, and when in use light from the object passes direct to the observer’s eye through an opening in the silvered surface of a prism. At the same time, light from the drawing paper and pencil is reflected by a second prism and by the silvered surface, so that the pencil appears superimposed on the object, which may thus be traced.
Polarization
Anisotropic substances exhibit different physical properties according to the direction along which they are examined. Such substances show more than one refractive index. The great majority of crystalline materials show birefringence. When a uniaxial crystal is placed with its optical axis horizontal to the stage and examined between crossed Nicols, then as the stage is rotated it will alternately shine bright (or coloured) and disappear. Through the 360° it becomes invisible (i.e. shows extinction) four times. The examination of crystals between crossed Nicols enables us to determine their crystal system (see ‘Calcium oxalate’ Chapter 42). The crystal is placed with its axis parallel to the longer diagonal of the polarizing Nicol. If the crystal belongs to the tetragonal system, the polarized light passes unchanged and on reaching the analyser is completely absorbed, the field appearing dark (i.e. extinction takes place). Conversely, monoclinic crystals show extinction only when the vertical axis makes an angle with the diagonal of the Nicol known as the extinction angle.
Electron microscopy
Much knowledge of the detailed structure of the living cell has only been made possible by the advent of the electron microscope. For the routine examination of vegetable drugs the light microscope with polarizing attachment is generally fully adequate, but scanning electron micrographs at a much lower magnification than the above can be extremely useful for depicting structural details not obvious with the light microscope, for example, maize starch and digitalis (Fig. 23.17).
PREPARATION OF DRUGS FOR MICROSCOPICAL EXAMINATION AND GENERAL USE OF REAGENTS
The following aims should be kept in mind for the microscopical examination of crude drugs.
Clearing, defatting and bleaching
Structures are frequently obscured by the abundance of cell contents, the presence of colouring matters and the shrinkage or collapse of the cell walls. Therefore, reagents are used for the removal of cell contents, for bleaching and for restoring as far as possible the original shape of the cell wall. If the microscopical examination is to be made from the section mounted in the clearing agent, the refractive index of the latter is important. It may be advisable to wash the section and mount in a different medium. The commonly used mountants glycerin, alcohol, carbolic acid, lactophenol, clove oil and Canada balsam all have some clearing effect. The following clearing and bleaching agents are particularly useful.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



