CHAPTER 9 Implantation and placentation
IMPLANTATION
Implantation involves the initial attachment of the trophoblastic wall of the blastocyst to the endometrial luminal epithelium. The trophoblast lineage gives rise to three main cell types in the human placenta: the syncytiotrophoblast, which forms the epithelial covering of the villous tree and is the main endocrine component of the placenta; the villous cytotrophoblast cells, which represent a germinative population that proliferate throughout pregnancy and fuse to generate the syncytiotrophoblast; and extravillous trophoblast cells, which are non-proliferative and invade the maternal endometrium. The first two cell lines can be seen from stages 4 and 5 onwards. The cytotrophoblast cells that form the mural and polar trophoblast are cuboidal, and covered externally with syncytial trophoblast (syncytiotrophoblast), a multinucleated mass of cytoplasm that forms initially in areas near the inner cell mass after apposition of the blastocyst to the uterine mucosa (see Fig. 8.10).
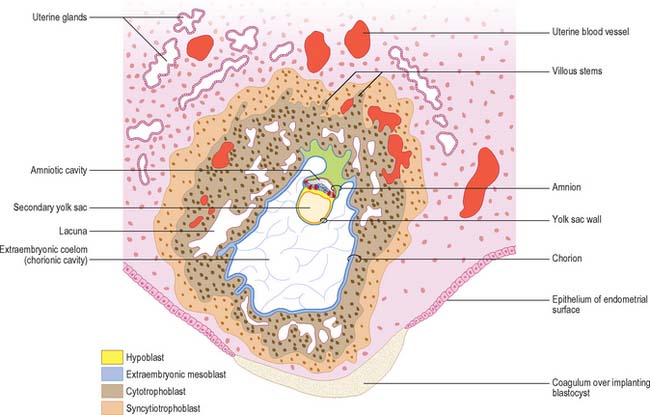
Implantation continues with erosion of maternal vascular endothelium and glandular epithelium, and phagocytosis of secretory products, until the blastocyst occupies an uneven implantation cavity in the stroma (interstitial implantation) (Fig. 9.1). In the early postimplantation phase, the maternal surface is resealed by re-epithelialization and the formation of a plug, which may contain fibrin. As the blastocyst burrows more deeply into the endometrium, syncytial trophoblast forms over the mural cytotrophoblast, but never achieves the thickness of the syncytial trophoblast over the embryonic pole.
Decidual differentiation is not evident in the stroma at the earliest stages of implantation, and it may not be until a week later that fully differentiated cells are present. During decidualization the interglandular tissue increases in quantity. It contains a substantial population of leukocytes (large granular lymphocytes, macrophages and T cells) distributed amongst large decidual cells: the most numerous are uterine natural killer (NK) cells which accumulate in the endometrium during the secretory phase of the cycle and persist until mid-pregnancy. Decidual cells are mesenchymally derived stromal cells which contain varying amounts of glycogen, lipid, and vimentin-type intermediate filaments in their cytoplasm. They are generally rounded, but their shape may vary depending on the local packing density. They may contain one, two or sometimes three nuclei and frequently display rows of club-like cytoplasmic protrusions enclosing granules. The cells are associated with a characteristic capsular basal lamina. Decidual cells produce a range of secretory products, including insulin-like growth factor binding protein 1 (IGF-BP1) and prolactin, which may be taken up by the trophoblast. These secretions probably play a role in the maintenance and growth of the conceptus in the early part of postimplantational development, and can be detected in amniotic fluid in the first trimester of pregnancy.
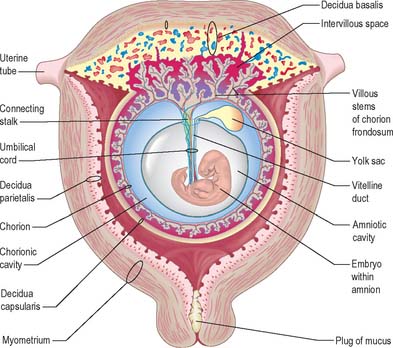
Extracellular matrix, growth factors and protease inhibitors produced by the decidua all probably modulate the degradative activity of the trophoblast and support placental morphogenesis and placental accession to the maternal blood supply. Once implantation is complete, distinctive names are applied to different regions of the decidua (Fig. 9.2). The part covering the conceptus is the decidua capsularis; that between the conceptus and the uterine muscular wall is the decidua basalis (where the placenta subsequently develops); and that which lines the remainder of the body of the uterus is the decidua parietalis. There is no evidence that their respective resident maternal cell populations exhibit site-specific properties.
DEVELOPMENT OF THE PLACENTA
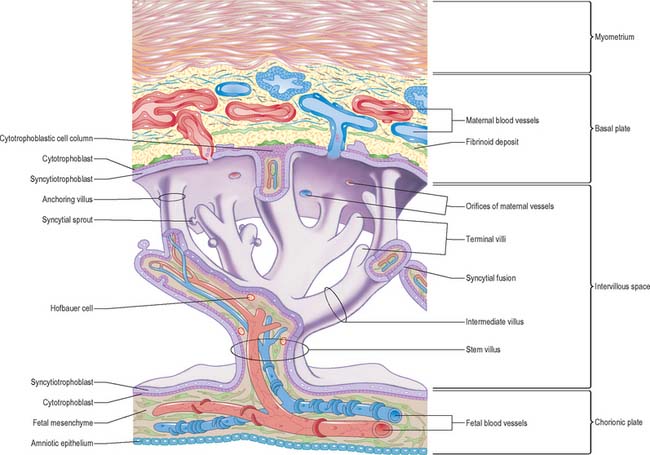
As the blastocyst implants, the syncytiotrophoblast invades the uterine tissues, including the glands and walls of maternal blood vessels (Fig. 9.1), and increases rapidly in thickness over the embryonic pole (Fig. 9.3). A progressively thinner layer covers the rest of the wall towards the abembryonic pole. Microvillus-lined clefts and lacunar spaces develop in the syncytiotrophoblastic envelope (days 9–11 of pregnancy) and establish communications with one another. Initially, many of these spaces contain maternal blood derived from dilated uterine capillaries and veins, as the walls of the vessels are partially destroyed. As the conceptus grows, the lacunar spaces enlarge, and become confluent to form an intervillous space.
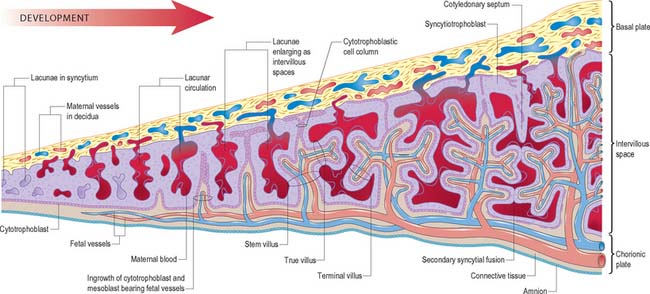
The projections of syncytiotrophoblast into the maternal decidua are called primary villi. They are invaded first with cytotrophoblast and then with extraembryonic mesenchyme (days 13–15) to form secondary placental villi. Fetal capillaries develop in the mesenchymal core of the villi. The cytotrophoblast within the villi continues to grow through the invading syncytiotrophoblast and makes direct contact with the decidua basalis, forming anchoring villi. Further cytotrophoblast proliferation occurs laterally so that neighbouring outgrowths meet to form a spherical cytotrophoblastic shell around the conceptus (Fig. 9.4). Lateral projections from the main stem villus form true and terminal villi.
With the onset of the embryonic heartbeat, a primitive circulation exists between the embryo and the yolk sac, succeeded later by that between the embryo and the placenta. The formed placenta is composed of a chorionic plate on its fetal aspect and a basal plate on the maternal aspect, and an intervening intervillous space containing villous stems with branches in contact with maternal blood (Fig. 9.4). Since maternal blood bathes the surfaces of the chorion which bound the intervillous space, the human placenta is defined as haemochorial. Different grades of fusion exist between the maternal and fetal tissues in many other mammals (e.g. epitheliochorial, syndesmochorial, endotheliochorial). The chorion is vascularized by the allantoic blood vessels of the body stalk, and so the human placenta is also termed chorio-allantoic (whereas in some mammals a choriovitelline placenta either exists alone or supplements the chorio-allantoic variety). In addition, the human placenta is defined as discoidal (in contrast to other shapes in other mammals) and deciduate because maternal tissue is shed with the placenta and membranes at parturition as part of the afterbirth.
Growth of the placenta
Expansion of the entire conceptus is accompanied by radial growth of the villi and, simultaneously, an integrated tangential growth and expansion of the trophoblastic shell. Eventually each villous stem forms a complex that consists of a single trunk attached by its base to the chorion, from which second and third order branches (intermediate and terminal villi) arise distally. Terminal villi are specialized for exchange between fetal and maternal circulations; each one starts as a syncytial outgrowth and is invaded by cytotrophoblastic cells, which then develop a core of fetal mesenchyme as the villus continues to grow. The core is vascularized by fetal capillaries (i.e. each villus passes through primary, secondary and tertiary grades of histological differentiation). The germinal cytotrophoblast continues to add cells that fuse with the overlying syncytium and so contribute to the expansion of the haemochorial interface. Terminal villi continue to form and branch within the confines of the definitive placenta throughout gestation, projecting in all directions into the intervillous space (Fig. 9.4).
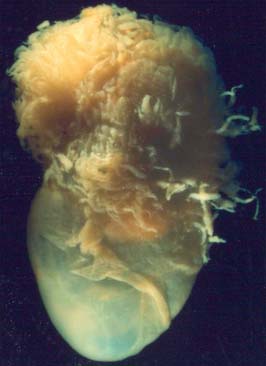
From the third week until about the second month of pregnancy, the entire chorion is covered with villous stems. They are thus continuous peripherally with the trophoblastic shell, which is in close apposition with both the decidua capsularis and the decidua basalis. The villi adjacent to the decidua basalis are stouter, longer and show a greater profusion of terminal villi. As the conceptus continues to expand, the decidua capsularis is progressively compressed and thinned, the circulation through it is gradually reduced, and adjacent villi slowly atrophy and disappear. This process starts at the abembryonic pole, and by the end of the third month, the abembryonic hemisphere of the conceptus is largely denuded. Eventually the whole chorion apposed to the decidua capsularis is smooth and is now termed the chorion laeve. In contrast, the villous stems of the disc-shaped region of chorion apposed to the decidua basalis increase greatly in size and complexity and the region is now termed the chorion frondosum (Fig. 9.3). The chorion frondosum and the decidua basalis constitute the definitive placental site (Fig. 9.2). Abnormalities in this process may account for the persistence of villi at abnormal sites on the chorionic sac and hence the presence of accessory or succenturiate lobes. At term, the placental diameter varies from 200 to 220 mm, the mean placental weight is 470 g, its mean thickness is 25 mm and the total villous surface area is 12–14 m2.
Chorionic plate
The chorionic plate is covered on its fetal aspect by the amniotic epithelium, on the stromal side of which is a connective tissue layer carrying the main branches of the umbilical vessels (Fig. 9.4 and Fig. 9.5). Subjacent to this is a diminishing layer of cytotrophoblast and then the inner syncytial wall of the intervillous space. The connective tissue layer is formed by fusion between the mesenchyme-covered surfaces of amnion and chorion: it is more fibrous and less cellular than Wharton’s jelly (of the umbilical cord), except near the larger vessels. The umbilical vessels radiate and branch from the cord attachment, with variations in the branching pattern, until they reach the bases of the trunks of the villous stems and then arborize within the intermediate and terminal villi. There are no anastomoses between vascular trees of adjacent stems. The two umbilical arteries are normally joined at, or just before they enter, the chorionic plate, by some form of substantial transverse anastomosis (Hyrtl’s anastomosis).
Basal plate
The basal plate, from fetal to maternal aspect, forms the outer wall of the intervillous space. The trophoblast and adjacent decidua are enmeshed in layers of fibrinoid and basement membrane-like extracellular matrix to form a complex junctional zone. In different places the basal plate may contain syncytium, cytotrophoblast or fibrinoid matrix, remnants of the cytotrophoblastic shell, and, at the site of implantation, areas of necrotic maternal decidua (the so-called Nitabuch’s stria) (Figs 9.4 and 9.5
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree