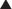Chapter 5 Impact of Age on Pharmacology
Fetus
A teratogen is defined as any agent that can cause malformations in a developing fetus.
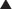 Size of drug
Size of drug• The larger the drug, the less likely it is to cross the placenta. Therefore larger drugs, such as proteins, are less likely to cross. However, one cannot assume that large drugs will not cross, as the placenta does have transporters that facilitate the transport of bulkier molecules.
• These transporters are typically used to transfer nutrients from the mother to the fetus and allow the fetus to transfer waste products of metabolism to the mother. Some of these transporters also facilitate transport of drugs, and in some cases, drugs can also inhibit the actions of these endogenous transporters. Examples of important substrates are listed in Box 5-1.
The risk of a drug causing harm to the fetus is categorized using the system in Table 5-1, which is largely based on the evidence (or, most commonly, lack of evidence) of harm.
 Note that relatively few drugs are at the extreme ends of the spectrum (either safe or absolutely contraindicated); this is typically because of a lack of good evidence. It is understandably difficult to conduct controlled trials in this population.
Note that relatively few drugs are at the extreme ends of the spectrum (either safe or absolutely contraindicated); this is typically because of a lack of good evidence. It is understandably difficult to conduct controlled trials in this population. The fact that most drugs fall in an intermediate area between safe and unsafe makes it difficult to decide whether a given drug should be used in pregnancy, and usually the decision depends on the risk-to-benefit assessment.
The fact that most drugs fall in an intermediate area between safe and unsafe makes it difficult to decide whether a given drug should be used in pregnancy, and usually the decision depends on the risk-to-benefit assessment. In some cases, not taking the medication may be more harmful to the pregnant patient (and fetus) than taking a medication with questionable risk.
In some cases, not taking the medication may be more harmful to the pregnant patient (and fetus) than taking a medication with questionable risk.TABLE 5-1 Risk Categories and Descriptions for Use of Drugs in Pregnancy
| Risk Category | Description |
|---|---|
| A | Controlled studies in humans fail to demonstrate risk to the fetus in the first trimester or later trimesters, and the possibility of fetal harm appears remote. |
| B | Either animal reproduction studies have not demonstrated a fetal risk but there are no controlled studies in pregnant women, or animal reproduction studies have shown an adverse effect (other than a decrease in fertility) that was not confirmed in controlled studies in women in the first trimester (and there is no evidence of risk in later trimesters). |
| C | Either studies in animals have revealed adverse effects on the fetus (teratogenic or embryocidal or other) and there are no controlled studies in women, or studies in women and animals are not available. Drugs should be given only if the potential benefit justifies the potential risk to the fetus. |
| D | There is positive evidence of human fetal risk, but the benefits in pregnant women may be acceptable despite the risk (e.g., if the drug is needed in a life-threatening situation or for a serious disease for which safer drugs cannot be used or are ineffective). |
| X | Studies in animals or human beings have demonstrated fetal abnormalities or there is evidence of fetal risk based on human experience or both, and the risk of the use of the drug in pregnant women clearly outweighs any possible benefit. The drug is contraindicated in women who are or may become pregnant. |
Timing of exposure is also important and is correlated with the stages of fetal development (Figure 5-1).
 First 14 days: Typically this is all or none, meaning that exposure results in death of the embryo or has no effect. The common feature of these two extremes is that they go undetected; if the embryo dies the patient does not realize she was ever pregnant.
First 14 days: Typically this is all or none, meaning that exposure results in death of the embryo or has no effect. The common feature of these two extremes is that they go undetected; if the embryo dies the patient does not realize she was ever pregnant. Days 14-60 (organogenesis): Exposure to teratogens at this stage may lead to death of the fetus or significant malformations affecting structure or function.
Days 14-60 (organogenesis): Exposure to teratogens at this stage may lead to death of the fetus or significant malformations affecting structure or function. Day 61 onward: Exposure does not typically result in major structural malformations unless blood supply has been disrupted. However, from this point onward the major concern is fetotoxicity.
Day 61 onward: Exposure does not typically result in major structural malformations unless blood supply has been disrupted. However, from this point onward the major concern is fetotoxicity.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree