Fig. 2.1.
Polymer-based detection system, providing amplification of the signal, with low background and excellent reproducibility.
♦
The “primary” antibody binds to the test antigen in the tissue section; the “secondary” or labeling antibody binds to the site of localization of the primary antibody
♦
Antibody is labeled, directly or indirectly, with a visible marker, usually an enzyme that acts upon a chromogen or substrate to give a colored product at the site of localization
♦
Several enzymes are employed, most often peroxidase or alkaline phosphatase
♦
Common chromogens are diaminobenzidine (DAB) and hydrogen peroxide for peroxidase and Fast Red for alkaline phosphatase; other commercial products available are brown, red, blue, black, and green
♦
IHC techniques have one principal aim in common – to attach the maximum amount of label at the site of localization of the antigen within the tissue with a minimum degree of nonspecific (background) binding
♦
Amplification methods have been developed to increase the amount of label deposited, increase the sensitivity of detection of antigen, and/or allow the use of antibody at higher dilutions
♦
The avidin–biotin conjugate (ABC) method was one of the early amplification methods, now largely replaced by polymer-linked antibodies that carry numerous enzymatically active sites; these reagents are widely available commercially and are generally of high quality, giving good results
♦
More than 30 years ago, it was shown that IHC methods are able to “stain” many antigens in routinely processed formalin-fixed paraffin-embedded (FFPE) tissues
♦
Subsequently, the range of “stains” that could be performed has increased enormously, as a result of two major factors: (1) the availability of literally thousands of monoclonal antibodies and (2) the advent of the antigen retrieval (AR) method [sometimes known as heat-induced epitope retrieval (HIER)] that to a large extent restores the antigenicity of many molecules after FFPE, permitting staining by IHC
Methods
General
♦
Immunohistochemical and in situ hybridization (ISH) methods, which are similar in practical application, have added a new dimension to the practice of histopathology, facilitating the specific demonstration of tissue, cell, and molecular components while conserving the ability to assess traditional morphologic criteria
♦
IHC usually is applied to an FFPE section as an aid to cell type recognition and diagnosis by the surgical pathologist
♦
There is growing recognition that results of IHC stains may be inconsistent between laboratories and even from day to day within a laboratory. The use of appropriate controls is therefore vital
♦
The recent extended use of IHC methods to detect prognostic and predictive markers has increased demands for standardization and for improved control systems
Staining Protocol
♦
In order to achieve optimal, reproducible results, it is necessary to monitor closely and control every step of the protocol, from tissue collection to interpretation of the final stained slide
♦
This is termed the “total test approach” (Table 2.1)
Table 2.1.
Immunohistochemistry Total Test Approach
Elements of testing process | Primary quality assurance issues |
|---|---|
Indications for IHC | Selection of appropriate stain(s) |
Specimen acquisition/preparation | Control of fixation time; antigen retrieval |
Staining protocol | Reagents: labeling method |
Analytic issues | Automation, qualifications of staff, proficiency testing of methods |
Results: validation, reporting | Controls, criteria for interpretation |
Interpretation; significance; reporting | Experience of pathologist; proficiency testing of reported results |
Indications for IHC
♦
Indications should be part of the practice of the institution and should be appropriately documented in the laboratory manual
♦
There are two broad approaches to selection of the stain(s), and neither one is exclusive of the other
Where the differential is very broad, as with a “tumor of unknown origin,” the decision may be to follow an algorithm
Or the surgical pathologist may formulate a “working” diagnosis and then select a small number of stains from the “test menu” in an attempt to confirm one option or exclude another
♦
Issues to consider include the number of stains that will be performed in total, the cost of such stains, reimbursement limits, and turnaround time (which impacts total hospital costs)
Specimen Acquisition/Preparation
♦
The fixative employed is critical; usually it is formalin
♦
Ideally tissue should be placed in the fixative immediately upon removal in order to minimize “cold ischemia” time during which proteins may degrade to unknown degree; this is considered important for RNA preservation and for proteins (IHC) and is critical for predictive markers where quantification is necessary
♦
The total time in the fixative including transport, wait time in pathology, and time of the “processor” should be considered
♦
Total fixation should not be less than 8 h and ideally not more than 24 h (though longer periods may suffice with the use of antigen retrieval)
♦
Guidelines have been published that cover fixation/processing protocols, and increasingly, there is a demand that ischemia and fixation times be recorded
♦
The above applies to FFPE tissues; IHC generally is also effective on frozen tissue or cytopathology preparations with alcohol fixation, although diffusion or loss of some antigens can occur
♦
Antigen retrieval (AR-HIER)
Has greatly broadened the utility of IHC on FFPE tissues
Used routinely on all FFPE tissues in many labs
Involves heating deparaffinized sections in buffer (usually citrate Ph6 or TRIS/EDTA pH9), usually for 20 min, usually in a microwave
Other buffers, times, and heating methods may be used for a minority of antibody/antigen stains where the standard method is not satisfactory
AR should be optimized and standardized “in house” for each antibody/antigen stain; control sections should be subjected to the same AR as test sections
For further details, consult the additional reading materials given at the end of the chapter
Staining Protocol
♦
Reagents
There are many commercial sources for both primary antibodies and labeling reagents
Basic rule for pathologists and technologists alike: “read the package insert”
Most primary antibodies are “monoclonal,” having certain advantages of specificity, but attention should be paid to clone designation (number) as well as to listed specificity (e.g., several antibody clones exist to ER, with very different performance characteristics) and the species of origin (usually mouse or rabbit) in order to be sure that an appropriate detection system is used
Polyclonal antibodies in some instances give better staining on FFPE but require careful specificity control (they, in fact, contain many different antibodies)
Two broad options:
“Home brew” – to purchase separately the primary and secondary (labeled) antibodies and conduct optimal titration/incubation time studies “in house” to establish the protocol
“RTU” – to purchase kits containing ready-to-use (RTU) reagents optimized by the manufacturer for both primary antibody and labeling system
Option B is technically simpler but still must be validated for performance with tissues fixed and processed “in house”
All reagents should be validated “in house” versus known positive and negative controls
♦
Protocols
The labeling method should be selected and validated with each of the primary antibodies
With kits, the labeling method is preselected but validation still is necessary
Labeling methods vary in ability for amplification, reproducibility, and ease of use
Currently, polymer-based reagents offer the best combination of ease of use, reliability, and cost
In general, results will be more consistent when a lab evaluates, selects, and uses the minimum number of different labeling systems that is required for the primary antibody menu and mouse monoclonal, rabbit monoclonal, and polyclonal antibodies. The more different the protocols, the less consistency
Double stain techniques employing two (or more) different primary antibodies are growing in popularity for improved assessment of complex staining patterns (e.g., CD20+CD3, B cell vs. T cell)
Analytical Issues
♦
Automations
There is a basic choice between manual protocols and automation
The choice is based upon the volume of IHC work and expertise of technologists
In general, automated methods offer better consistency and control
Automated systems may be “closed,” that is, the labeling system employed is defined by the instrument, or “open,” where the user has the option of selecting and optimizing the whole protocol. The latter approach requires considerable “in house” technical experience
♦
Sensitivity and specificity
Sensitivity in IHC is applied as a descriptor of the extent to which the primary antibody can be diluted and still achieve target recognition
This usage differs from that in clinical pathology where sensitivity describes the ability of a test to detect positive cases of a disease within a population having the disease
Specificity is used to describe the extent to which an antibody binds only its intended target (monoclonal antibodies have good specificity; polyclonal antibodies less)
Sensitivity and specificity are, however, sometimes used loosely to describe the extent to which an IHC stain identifies a particular cell/tumor type (e.g., an S-100 stain has a high sensitivity for melanoma but low specificity; the S-100 antibody itself may be both sensitive and specific for S-100 protein; it is the distribution of the antigen that is addressed in this usage)
♦
Qualifications of staff
This critically affects the ability to perform good IHC staining
There is no substitute for relevant and proper experience
In addition to the histotechnology certification process, the American Society for Clinical Pathology (ASCP, Chicago, IL, USA) operates an immunohistochemistry qualification program
Comparable qualification and training exist in the European Union and elsewhere, but presently there is no international reciprocity
♦
Intra- and interlaboratory proficiency testing of procedures
Participation in an external proficiency program is highly recommended
The College of American Pathologists (CAP) provides such a program in the USA, by subscription
The United Kingdom National External Quality Assessment Scheme for Immunohistochemistry (UK-NEQAS-ICC, http://www.ukneqas.org.uk) and NordiQC (www.nordiqc.org) offer more extensive programs in Europe and elsewhere, also including the evaluation of interpretation by the pathologist. Similar programs are beginning in Canada, Australia, and China
Guidelines for running an IHC lab are provided by the CAP accreditation program and CLSI (http://www.clsi.org)
Results: Validation/Reporting
♦
Controls/criteria for interpretation
For a comprehensive review of controls, consult the bibliography at the end of the chapter
As a minimum, each batch run for a particular stain should include an “in house” positive control and a negative control (if a panel is run, then with experience, different stains in the panel may serve as negative controls for other panel members stained by the same protocol); in the USA, the CAP has determined the use of “negative controls” as discretionary if polymer-based systems are used (but not for avidin–biotin because of known “false positives”)
The control section should have been fixed and processed in a manner identical to the test section
Tissue microarrays (or sausage blocks) are useful when a new antibody is being validated (allows rapid and identical testing of multiple cases) but are not necessary and are even wasteful of tissues, for batch controls
The results in the control section provide the basis for interpreting the staining pattern observed in the test section
Note that for many stains, internal positive controls exist within the tissue; for example, residual normal plasma cells serve as controls for Ig staining of lymphomas and residual normal breast ducts as controls when staining for ER
It has been proposed that such internal controls, when quantified, may provide the basis for quantitative IHC (quantifiable internal reference standards), analogous to the ELISA method, used to measure proteins in the clinical laboratory (see bibliography)
Turnaround time (TAT): overnight incubations may fit well into the usual workload patterns of histology labs. If needed, IHC staining protocols may be designed to be completed in 90 min or less with the availability of the cut FFPE section
In search of short TAT for the total test, fixation should not be reduced below 8 h
Interpretation, Significance, and Reporting
♦
As with surgical pathology in general, the reliability of the IHC interpretation increases with the experience of the pathologist
♦
In general, the diagnosis of malignancy should be made by histological examination, while the use of immunohistochemistry, with rare exceptions, should be restricted to further characterize and classify the lesion in question
♦
IHC is best used to advantage in the context of a carefully formulated differential diagnosis that integrates clinical history, lesion topography including imaging study characteristics, specimen type, and thorough morphologic assessment
♦
Image analysis techniques are increasingly employed to assist the pathologist in interpretation, especially in rare event detection (micrometastases) and in semiquantitative analysis of HER2, ER, and PR scores or proliferation rates (Ki67)
♦
The report should include as a minimum the usual patient demographics, the type of specimen tested (e.g., frozen, FFPE), antibodies employed (with clone designations as appropriate), results for all of the antibodies used (both positive and negative), and significance of the findings
♦
Reagent sources, exact protocols, and AR methods need not be part of the report but should be recorded in the laboratory log, for reference if needed
Applications
Tumor Diagnosis and Classification
♦
Historically IHC has usually been employed for cell type and tumor identification
♦
These remain the most common uses today, with several hundred antibodies in routine use
♦
In this context, antibodies are chosen that recognize structural proteins or cell products that have a distribution restricted to a particular cell (tumor) lineage
♦
All surgical pathology books today include discussion of IHC markers along with histopathology; this also is true of the present text where IHC features are briefly described for each tumor type
♦
In addition, the remainder of this chapter presents a concise review of the major diagnostic applications of IHC, with an emphasis on tumor diagnosis and classification
♦
A more focused or extensive discussion may be found in subsequent chapters and other bibliography where contextual differential diagnostic considerations dictate either more limited or more extensive panels
Tumor of Unknown Origin: Anaplastic Malignancies
♦
A separate chapter is devoted to the use of IHC in unknown or anaplastic tumors
♦
The use of a “decision tree” approach is discussed, together with other aspects of the rationale for antibody selection
♦
The anticipated findings with commonly used panels are described
Prognostic and Predictive Markers (Also Termed “Companion Diagnostics”)
♦
A “prognostic” marker addresses outcome regardless of therapy; a “predictive marker” addresses outcome with respect to a specified treatment
♦
IHC methods have been adapted for the identification of the degree of expression by tumors of certain molecules of known prognostic significance (e.g., ER and PR in breast cancer; Ki-67 or MIB-1 as a marker of proliferation)
♦
The demonstration of HER2 expression in breast or gastric cancer is an example of a predictive marker, of value in selecting patients likely to respond to Herceptin therapy
♦
Performance of IHC “assays” for these markers requires rigorous adherence to test protocols (for reproducibility), while interpretation requires some form of quantitative assessment by reference to standard controls and strict scoring guidelines
♦
With numerous other companion diagnostics (prognostic and predictive markers) currently in development, accurate quantitative IHC methods are being explored using quantifiable internal reference standards and image analysis as described in the appended bibliography
Identification of Focal Invasion
♦
IHC staining for intact layers of basal type cells, in breast and prostate, is of value in distinguishing in situ carcinoma or intraepithelial neoplasia from early and focal invasion
NOTE: Antibodies in italics are the most useful for differential diagnosis. Exceptions exist for virtually all typically expected results; therefore, panels should be used whenever possible to identify a diagnostically reliable pattern of antigen expression.
♦
Loss or disruption of the basal layer may be demonstrated with different antibodies, including high molecular weight keratins, smooth muscle myosin, or p63 nuclear staining. The basement membrane itself may be assessed with antibodies to laminin and collagen type IV
♦
This is an area where double or triple stains are especially useful for interpretation
♦
The result descriptions refer to typically expected immunoreactivity patterns. Refer to corresponding chapters or other sources for additional markers that may aid in specific atypical situations
Identification of Micrometastases
♦
Although still under study, many investigators feel that the identification of micrometastases (small numbers of cancer cells) in sentinel or draining lymph nodes has value for prognosis and therapeutic decisions
♦
It has clearly been shown that IHC allows recognition of tumor micrometastases that are not identified on H&E
♦
Automated image analysis systems are especially suited to this purpose (rare event detection)
♦
Keratin cocktails are used for the detection of carcinoma cells and cocktails of S-100 with HMB 45 or melan-A for melanoma cells
♦
Similar methods also are applied to bone marrow and peripheral blood detection, where the data are less clear
Identification of Infectious Agents
♦
There is an extensive literature on the detection of bacteria, parasites, and viruses by IHC
♦
Current applications include HPV subtypes in dysplasia of the cervix and squamous carcinoma, Toxoplasma in brain biopsies, and H. pylori in gastric biopsies. The identification of Epstein–Barr virus infection by IHC and in situ hybridization has become commonplace in the study of lymphomas
Head and Neck
Upper Respiratory Tract
Infection
♦
IHC antibodies to herpes simplex virus (HSV1 and HSV2) show nuclear and cytoplasmic staining of infected squamous cells
Squamous Cell Carcinoma
♦
IHC may become necessary for the diagnosis of squamous carcinoma when diffusely poor differentiation precludes recognition of keratin production by routine light microscopy
Verification Panel (Table 2.2)
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm
♦
p63 stains the tumor cell nuclei; p40 (desmoglein) also stains nuclei of squamous cells with indications of higher sensitivity and specificity
♦
Lymphoepithelial carcinoma shows scattered single cells or nests of HMWK-positive cells admixed with CD45-positive tumor-associated lymphoid cells
Table 2.2.
Head and Neck Small Round Blue Cell Tumor Differential Diagnosis
Synaptophysin | S-100 | Pankeratin | Muscle markers a | CD45 | |
|---|---|---|---|---|---|
Poorly differentiated squamous cell | |||||
carcinoma | – | − | + | – | −b |
Sinonasal undifferentiated carcinoma | – | − | + | − | − |
Neuroendocrine carcinoma | + | − | + | − | − |
Rhabdomyosarcoma | – | − | − | + | − |
Lymphoma | − | − | − | − | + |
Olfactory neuroblastoma | + | − | − | − | − |
Melanoma | − | + | − | − | − |
Differential Diagnosis
♦
Poorly differentiated adenocarcinoma is positive for pankeratin and CEA and negative for HMWK and p63 (nuclear)
♦
Pseudoepitheliomatous hyperplasia is a malignant-appearing reactive change overlying another lesion (including granular cell tumor and lymphoma) or found adjacent to ulcers. IHC is not helpful to differentiate reactive versus neoplastic squamous cells, but it can identify the underlying lesion; granular cell tumor is positive for S–100 and lymphoma for CD45
Small and Large Cell Neuroendocrine Carcinoma
Verification Panel (Table 2.2)
♦
Pankeratin shows dot-like or diffuse cytoplasmic staining
♦
Chromogranin, synaptophysin, and NSE show variable tumor cell cytoplasmic staining
♦
CD56 (membranocytoplasmic) is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology and keratin staining. CD56 immunoreactivity ought not to be the sole criterion to characterize a tumor as neuroendocrine
♦
S-100 can be focally positive in the tumor cell nuclei and cytoplasm (19%)
Differential Diagnosis
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for pankeratin, synaptophysin, and chromogranin
♦
Melanoma is positive for S-100, HMB 45, and melan–A and negative for pankeratin, synaptophysin, chromogranin, and CD56
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear) and Myo–D1 (nuclear) and negative for keratin, synaptophysin, and chromogranin
♦
Ewing sarcoma/peripheral neuroectodermal tumor is positive for Fli–1 (nuclear) and CD99 (membranous); some cases are focally positive for pankeratin (9%) and CD56 (9%) and negative for synaptophysin and chromogranin
♦
Olfactory neuroblastoma is positive for CD56, chromogranin, synaptophysin, and vimentin and negative for pankeratin
Mucosal Malignant Melanoma
Verification Panel (Table 2.2)
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
Melanocytic markers (HMB 45, melan-A) stain the tumor cell cytoplasm
♦
Rare cases show focal pankeratin-positive cytoplasm (3%)
Differential Diagnosis
♦
Carcinoma is diffusely positive for pankeratin; some cases are focally positive for S-100 (19%) and negative for HMB 45 and melan–A
♦
Small cell carcinoma is positive for pankeratin, chromogranin, synaptophysin, CD56, and S-100 (19%) and negative for HMB 45 and melan–A
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), and Myo–D1 (nuclear); focally positive for S-100; and negative for HMB 45 and melan–A
Rhabdomyosarcoma
Verification Panel (Table 2.2)
♦
Desmin and MSA stain the tumor cell cytoplasm
♦
Myogenin and Myo-D1 stain the tumor cell nuclei
♦
S-100 can be focally positive in the tumor cell nuclei and cytoplasm (12%)
Differential Diagnosis
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Ewing sarcoma/peripheral neuroectodermal tumor is positive for Fli–1 (nuclear) and CD99 (membranous) and negative for desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Mucosal malignant melanoma is positive for S-100, HMB 45, and melan–A and negative for desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Neuroblastoma and retinoblastoma are positive for CD56, chromogranin, and synaptophysin and negative for desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Undifferentiated carcinoma is positive for pankeratin and negative for desmin, myogenin (nuclear), and Myo–D1 (nuclear)
Angiosarcoma
Verification Panel
♦
CD31, CD34, and factor VIII stain the tumor cell cytoplasm; benign endothelial cells are also positive
♦
Fli-1 stains the tumor and benign endothelial cell nuclei
♦
Pankeratin can be focally positive in the tumor cell cytoplasm (17%)
Differential Diagnosis
♦
Poorly differentiated carcinoma is diffusely positive for pankeratin and negative for CD31, CD34, factor VIII, and Fli–1 (nuclear)
♦
Fibrosarcoma is positive for vimentin and negative for CD31, CD34, factor VIII, and Fli–1 (nuclear)
Kaposi Sarcoma
Verification Panel
♦
CD31, CD34, D2-40, and factor VIII stain the tumor cell cytoplasm; benign endothelial cells are also positive
♦
Fli-1 and HHV8 stain the tumor cell nuclei. Benign endothelial cells are also positive for Fli-1
Differential Diagnosis
♦
Poorly differentiated carcinoma is positive for pankeratin and negative for CD31, CD34, factor VIII, Fli–1 (nuclear), and HHV8 (nuclear)
♦
Angiosarcoma and lobular capillary hemangioma (pyogenic granuloma) are positive for CD31, CD34, factor VIII, and Fli-1 (nuclear) and negative for HHV8 (nuclear)
♦
Fibrosarcoma is negative for CD31, CD34, factor VIII, Fli–1 (nuclear), and HHV8 (nuclear)
Langerhans Cell Histiocytosis
Verification Panel
♦
CD1a and langerin stain the tumor cell membranes and cytoplasm
♦
S-100 stains the tumor cell nuclei and cytoplasm
Differential Diagnosis
♦
Histiocytic reaction is positive for S-100 and CD68 and negative for CD1a and langerin
♦
Reed–Sternberg cells of Hodgkin lymphoma (classic types) are positive for CD15 and CD30 and negative for CD1a and CD45; LP type is CD45 positive
Lymphoma (Table 2.2)
♦
Most l ymphomas show CD45 membranocytoplasmic staining. Most are B cells with CD20 membranous staining. Refer to the section and chapter on lymph node for further discussion
Nasopharynx
♦
Salivary gland and brain tumors (e.g., meningioma and pituitary adenoma) can involve the nasopharynx; see the appropriate sections for further discussion
Nasopharyngeal Carcinoma
♦
Differentiated and undifferentiated types are based on morphology
♦
IHC is unnecessary for the diagnosis of squamous carcinoma except when diffusely poorly differentiated
Verification Panel
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm
♦
p63 stains the tumor cell nuclei
♦
EBV LMP1 stains the membrane/cytoplasm, and EBV EBER (in situ hybridization) stains the nuclei in scattered tumor cells, more commonly in the undifferentiated tumors
♦
The undifferentiated lymphoepithelial type shows scattered single or nests of HMWK-positive cells admixed with CD45-positive tumor-associated lymphoid cells
Differential Diagnosis
♦
Poorly differentiated adenocarcinoma is positive for CEA and negative for HMWK and p63 (nuclear)
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for pankeratin
Sinonasal Undifferentiated Carcinoma
Verification Panel (Table 2.2)
♦
Pankeratin and CK7 stain the tumor cell cytoplasm
♦
NSE, synaptophysin, chromogranin, and S-100 show variable cytoplasmic staining
Differential Diagnosis
♦
Undifferentiated nasopharyngeal carcinoma is positive for HMWK, EBV EBER, EBV LMP1, and p63 (nuclear)
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for pankeratin
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for pankeratin
♦
Neuroendocrine carcinoma is positive for pankeratin, chromogranin, synaptophysin, and CD56
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), and Myo–D1 (nuclear) and negative for pankeratin
♦
Ewing sarcoma/peripheral neuroectodermal tumor is positive for Fli–1 (nuclear) and CD99 (membranous), and some cases are focally positive for pankeratin (30%)
♦
Olfactory neuroblastoma is positive for CD56, chromogranin, synaptophysin, and vimentin and negative for pankeratin
Sinonasal Adenocarcinoma, Intestinal Type
Verification Panel
♦
CK7 (60%), CK20, villin, and CEA stain the tumor cell cytoplasm
♦
CDX2 stains the tumor cell nuclei
Differential Diagnosis
♦
Sinonasal adenocarcinoma, nonintestinal type, is positive for CK7 and negative for CK20, villin, and CDX2 (nuclear)
♦
Adenoid cystic carcinoma contains myoepithelial cells that are positive for calponin, SMA, and p63 (nuclear)
♦
Metastatic colon carcinoma is positive for CK20, CEA, and CDX2 (nuclear) and negative for CK7
Olfactory Neuroblastoma (Esthesioneuroblastoma)
Verification Panel (Table 2.2)
♦
Synaptophysin, chromogranin, NSE, and vimentin stain the tumor cell cytoplasm
♦
CD56 stains the tumor cell membranes and cytoplasm
♦
S-100 stains the cytoplasm and nuclei of the spindled sustentacular cells surrounding the tumor cells in the nested or paraganglioma variant
♦
Pankeratin can be focally positive (35%)
♦
EMA is negative
Differential Diagnosis
♦
Neuroendocrine carcinoma is positive for chromogranin, synaptophysin, CD56, EMA, and pankeratin and negative for vimentin
♦
Mucosal malignant melanoma is positive for S-100, HMB 45, melan–A, and vimentin and negative for chromogranin and synaptophysin
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), Myo–D1 (nuclear), and vimentin and negative for synaptophysin and chromogranin
♦
Lymphoma is positive for CD45, vimentin, and CD3 or CD20 and negative for synaptophysin and chromogranin
♦
Sinonasal undifferentiated carcinoma is positive for pankeratin and negative for vimentin
♦
Ewing sarcoma/peripheral neuroectodermal tumor is positive for Fli–1 (nuclear), CD99 (membranous), and vimentin; some cases are focally positive for keratin and synaptophysin and negative for chromogranin
Mesenchymal Chondrosarcoma
Verification Panel (Fig. 2.2)
♦
CD99 stains the tumor cell membranes (specific) and cytoplasm (nonspecific)
♦
Desmin, CD56, CD57, and NSE show variable cytoplasmic staining
♦
S-100 stains the cytoplasm and nuclei of the cartilaginous component
♦
Keratins and EMA are negative
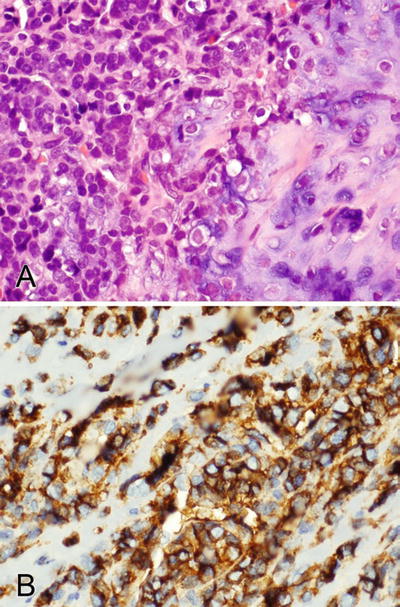
Fig. 2.2.
(A, B) CD99 stains the membranes (specific finding) and cytoplasm (nonspecific) of a mesenchymal chondrosarcoma.
Differential Diagnosis
♦
Neuroendocrine carcinoma is positive for chromogranin, synaptophysin, EMA, CD56 and pankeratin and negative for CD99 (membranous)
♦
Mucosal malignant melanoma is positive for S-100, HMB 45, and melan–A and negative for CD99 (membranous)
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), and Myo–D1 (nuclear) and negative for CD99 (membranous)
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for desmin. Some types are positive for CD56, CD57, or CD99 (membranous)
♦
Sinonasal undifferentiated carcinoma is positive for pankeratin and negative for vimentin and CD99 (membranous)
♦
Ewing sarcoma/peripheral neuroectodermal tumor is positive for Fli–1 (nuclear) and CD99 (membranous)
♦
Synovial sarcoma is positive for pankeratin, EMA, CD99 (membranous), and S-100
Other Mesenchymal Tumors
♦
Other nasopharyngeal mesenchymal tumors include nasal angiofibroma, solitary fibrous tumor/hemangiopericytoma, fibromatosis, osteosarcoma, chondrosarcoma, Ewing sarcoma, malignant peripheral nerve sheath tumor, fibrosarcoma, and Kaposi sarcoma. Spindle cell carcinoma and melanoma must be considered in the differential diagnosis. Refer to the mesenchymal section of this chapter and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common Western primary types are CD20-positive diffuse large B-cell, follicular cell, and extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT). NK-/T-cell lymphoma is positive for CD2, CD56, and CD3e (cytoplasmic) and is the most common nasal lymphoma in China. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the nasopharynx are kidney, lung, breast, thyroid, and prostate. Refer to Chapter 3 for further discussion
Oropharynx
♦
The diagnosis of the numerous oral jaw tumors is based on morphology. These include odontogenic, mesenchymal, and salivary gland neoplasms
Granular Cell Tumor
♦
This tumor can provoke a marked pseudoepitheliomatous hyperplasia that simulates invasive squamous cell carcinoma. The granular cell tumor cells must be identified around and below the reactive squamous nests
Verification Panel
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
CD68 and inhibin stain the tumor cell cytoplasm
♦
Epithelial markers (various keratins and EMA) are negative
Differential Diagnosis
♦
Carcinoma is positive for pankeratin; some cases are positive for S-100 (19%) and negative for inhibin
♦
Histiocytic inflammation is positive for S-100 and CD68 and negative for inhibin
♦
Paraganglioma is positive for chromogranin and synaptophysin and negative for CD68 and inhibin. S-100 stains the spindled sustentacular cells surrounding the tumor cell nests
Rhabdomyoma
Verification Panel
♦
MSA and desmin stain the tumor cell cytoplasm
♦
Myogenin and Myo-D1 stain the tumor cell nuclei
♦
S-100 focally stains the tumor cell nuclei and cytoplasm
♦
Epithelial markers (various keratins and EMA) are negative
Differential Diagnosis
♦
Granular cell tumor is diffusely positive for S–100 and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Paraganglioma is positive for chromogranin and synaptophysin and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear). S-100 stains the spindled sustentacular cells surrounding the tumor cell nests
♦
Histiocytic reaction is positive for S-100 and CD68 and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Carcinoma is positive for pankeratin and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear)
Other Mesenchymal Tumors
♦
Other laryngeal mesenchymal tumors include osteosarcoma, mesenchymal chondrosarcoma, rhabdomyosarcoma, Ewing sarcoma, and chondrosarcoma. Refer to the mesenchymal section of this chapter and Chapter 3 for further discussion. Spindle cell carcinoma and melanoma must be considered in the differential diagnosis
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common Western primaries are CD20-positive diffuse large B-cell, follicular cell, Burkitt, and extranodal marginal-zone B-cell lymphomas of mucosa-associated lymphoid tissue (MALT) lymphomas. NK-/T-cell lymphoma occurs rarely in the oropharynx and is positive for CD2, CD56, and CD3e (cytoplasmic). Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the oropharynx are breast, lung, prostate, and kidney. Refer to Chapter 3 for further discussion
Salivary Gland
♦
IHC is usually unnecessary for the diagnosis of salivary gland tumors
Adenoid Cystic Carcinoma
Verification Panel
♦
CEA, EMA, and keratin stain the cytoplasm of the ductal component
♦
SMA, SMMHC, calponin, and keratin stain the cytoplasm of the myoepithelial cell component
Differential Diagnosis
♦
Polymorphous low-grade carcinoma, mucoepidermoid carcinoma, and basaloid squamous carcinoma lack a myoepithelial component and are negative for SMA, SMMHC, and calponin
♦
Basal cell adenoma and epithelial–myoepithelial carcinoma show the same IHC pattern and must be differentiated by morphology
Salivary Duct Carcinoma
Verification Panel (Fig. 2.3)
♦
GCDFP-15 stains the tumor cell cytoplasm
♦
HER2 stains the tumor cell membranes
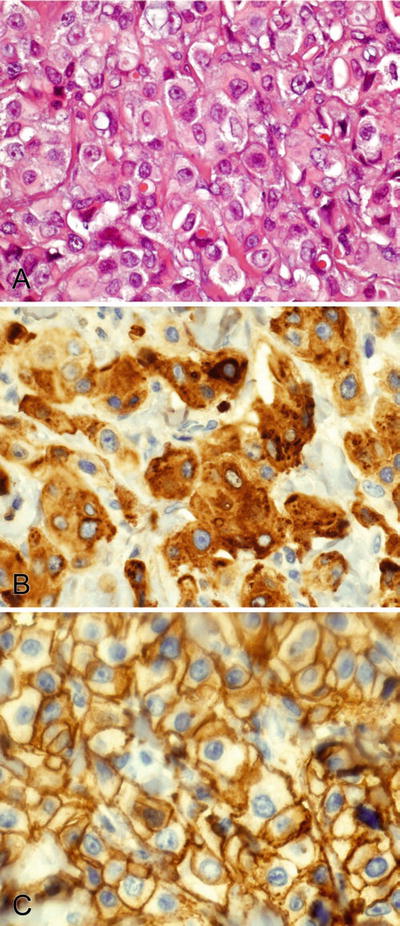
Fig. 2.3.
(A–C) GCDFP-15 stains the cytoplasm and HER2 stains the membranes of a salivary duct carcinoma.
Differential Diagnosis
♦
High-grade mucoepidermoid carcinoma is positive for GCDFP–15 (17%) and HER2 (21%) in some cases and shows squamous differentiation
♦
Poorly differentiated squamous cell carcinoma is often positive for HER2 (60%) and negative for GCDFP–15
♦
Acinic cell carcinoma is sometimes positive for GCDFP-15 (42%) and negative for HER2
♦
Metastatic breast carcinoma must be identified by clinical history
Epithelial–Myoepithelial Carcinoma
Verification Panel
♦
EMA and keratin stain the cytoplasm of the ductal component. Vimentin is negative
♦
SMA, SMMHC, calponin, vimentin, and pankeratin stain the cytoplasm and p63 the nuclei of the myoepithelial cell component. S-100 and GFAP show variable cytoplasmic staining
Differential Diagnosis
♦
Mucoepidermoid carcinoma, acinic cell carcinoma, sebaceous carcinoma, and metastatic renal cell carcinoma lack a myoepithelial component and are negative for SMA, SMMHC, and calponin
Myoepithelial Carcinoma
Verification Panel
♦
Pankeratin, SMA, calponin, SMMHC, and vimentin stain the tumor cell cytoplasm
♦
p63 stains the tumor cell nuclei
♦
S-100 and GFAP show variable cytoplasmic staining
Differential Diagnosis
♦
Poorly differentiated carcinoma is positive for pankeratin; some types (squamous carcinoma) are positive for p63 (nuclear) and negative for SMA, calponin, SMMHC, and vimentin
♦
Leiomyosarcoma is positive for SMA, MSA, desmin, and calponin, and some are focally positive for keratin (40%) and p63 (nuclear) (23%)
♦
Nerve sheath tumor is positive for S-100 and negative for SMA, calponin, SMMHC, and p63 (nuclear)
♦
Melanoma is positive for S-100, HMB 45, and melan–A and negative for SMA, calponin, SMMHC, and p63 (nuclear)
♦
Synovial sarcoma is positive for CD99, bcl–2, EMA, pankeratin, S-100, calponin, and calretinin and negative for p63 (nuclear)
Mesenchymal Tumors
♦
Salivary gland mesenchymal tumors include hemangioma, lipoma, neurofibroma, inflammatory myofibroblastic tumor, malignant peripheral nerve sheath tumor, and rhabdomyosarcoma. Refer to the mesenchymal section of this chapter and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. Most lymphomas involving the salivary glands are CD20-positive extranodal marginal-zone B-cell lymphoma of mucosa-associated lymphoid tissue (MALT), diffuse large B-cell, and follicular lymphoma. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the salivary glands are skin (squamous carcinoma and melanoma), nasopharynx, lung, breast, kidney, and colorectal. Refer to Chapter 3 for further discussion
Thyroid Gland
Papillary Thyroid Carcinoma
Verification Panel
♦
Thyroglobulin stains the tumor cell cytoplasm and colloid
♦
TTF-1 stains the tumor cell nuclei; benign follicular and C cells are also positive
♦
HMWK (CK34βe12, CK5/6) stains the areas of squamous metaplasia within the tumor
♦
Pankeratin stains the tumor cell cytoplasm
♦
Follicular variant of papillary carcinoma is diffusely positive for CK19
Differential Diagnosis (Table 2.3)
♦
Follicular adenoma and carcinoma are negative or weak/focal for CK19. Various markers have been studied to differentiate papillary and follicular neoplasms, but none are sensitive or specific
♦
Galectin-3, HBME1, and CK19 may be helpful in small specimens to identify malignant follicular proliferations since these markers tend to be negative in benign follicular thyroid lesions
Table 2.3.
Thyroid and Parathyroid Gland Tumor Differential Diagnosis
Thyroglobulin | TTF-1 | TTF-1 calcitonin, CEA | Chromogranin | Parathyroid hormone | |
|---|---|---|---|---|---|
Papillary carcinoma | + | + | − | − | − |
Follicular neoplasms | + | + | − | − | − |
Medullary carcinoma | − | + | + | + | − |
Parathyroid neoplasms | − | − | − | + | + |
Follicular Adenoma and Carcinoma
♦
Immunostains do not at this time definitively differentiate these tumors; however, galectin-3, HBM1, and CK19 have been demonstrated to be expressed more significantly in malignant than benign follicular lesions. Vascular markers (CD31, CD34) help identify lymphovascular invasion
Verification Panel
♦
Thyroglobulin stains the tumor cell cytoplasm and colloid
♦
TTF-1 stains the tumor cell nuclei; benign follicular and C cells are also positive
♦
Pankeratin stains the tumor cell cytoplasm
Differential Diagnosis (Table 2.3)
♦
Follicular variant of papillary carcinoma can be diffusely positive with CK19, while follicular carcinoma is negative or weak/focal
♦
An intrathyroid parathyroid gland or adenoma is positive for parathyroid hormone and negative for TTF–1 (nuclear) and thyroglobulin
Hürthle Cell Neoplasms
♦
This morphologic subtype of follicular tumors behaves more aggressively; it should be considered in a patient with lung or bone metastases with a history of thyroid disease
♦
These tumors show weaker thyroglobulin expression than the other follicular neoplasms and similar TTF-1 (nuclear) and pankeratin staining
♦
In addition, these tumors show CEA cytoplasmic staining
Differential Diagnosis
♦
The clear cell variant of Hürthle cell tumors must be distinguished from metastatic renal cell carcinoma (RCC). RCC is positive for RCC Ma, CD10, and PAX8 and negative for TTF–1 (nuclear) and thyroglobulin
Medullary Thyroid Carcinoma
♦
Medullary carcinoma can have spindled, epithelioid, plasmacytoid, nested, and follicular morphology
Verification Panel (Fig. 2.4)
♦
Calcitonin, CEA, chromogranin, and synaptophysin stain the tumor cell cytoplasm
♦
TTF-1 stains the tumor cell nuclei; benign follicular and C cells are also positive
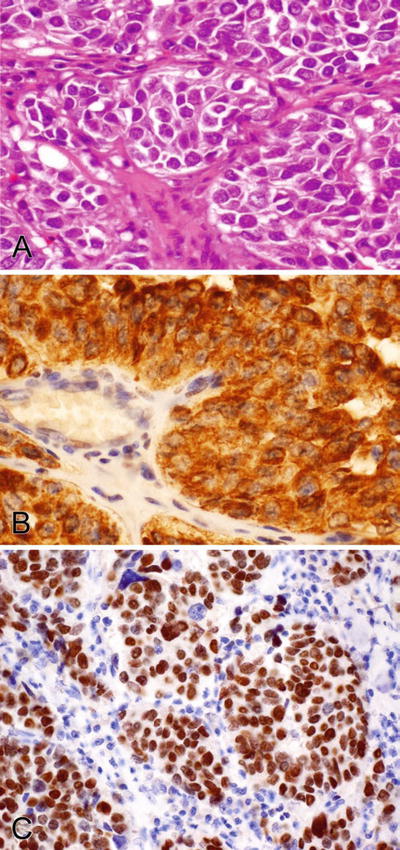
Fig. 2.4.
(A–C) Calcitonin stains the cytoplasm and TTF-1 stains the nuclei of a thyroid medullary carcinoma.
Differential Diagnosis (Table 2.3)
♦
Follicular and papillary neoplasms are positive for thyroglobulin and TTF-1 (nuclear) and negative for calcitonin, chromogranin, and synaptophysin
♦
Carotid body tumors or paraganglioma are positive for chromogranin and synaptophysin and negative for calcitonin and TTF–1 (nuclear)
Undifferentiated (Anaplastic) Thyroid Carcinoma
♦
This undifferentiated carcinoma stains poorly with IHC. Better-differentiated areas are helpful for morphologic diagnosis and focal expression with IHC testing
Verification Panel (Fig. 2.5)
♦
Pankeratin and vimentin stain the tumor cell cytoplasm focally
♦
TTF-1 (nuclear, 25%) and thyroglobulin (cytoplasm, 27%) stain the tumor cells only focally; both should be used to increase sensitivity
♦
Monoclonal CEA and HMWK stain the tumor cell cytoplasm focally
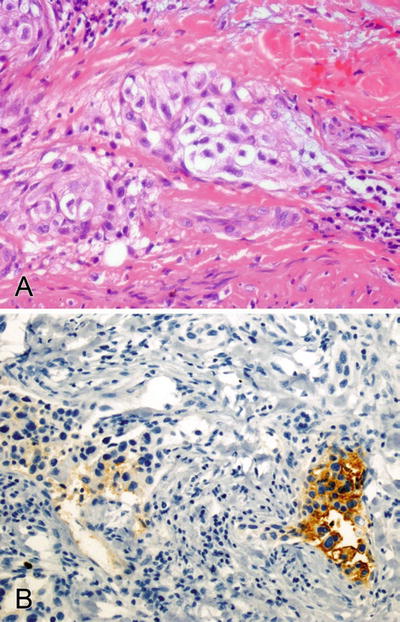
Fig. 2.5.
(A, B) Thyroglobulin focally stains the cytoplasm of an anaplastic thyroid carcinoma.
Differential Diagnosis
♦
Because of the varied morphology (spindled, pleomorphic, giant cell, small cell, and lymphoepithelial) of anaplastic thyroid carcinoma, many other poorly differentiated tumors must be considered including sarcoma, lymphoma, and metastatic carcinoma. Thyroglobulin and TTF–1 are negative in these tumors. Refer to Chapter 3 for further discussion
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for keratin, thyroglobulin, and TTF–1 (nuclear)
Poorly Differentiated Squamous Carcinoma
Verification Panel
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm
♦
p63 stains the nuclei
Differential Diagnosis
♦
Medullary thyroid carcinoma is positive for TTF–1 (nuclear) and calcitonin and negative for p63
♦
Anaplastic thyroid carcinoma is focally positive for thyroglobulin and/or TTF–1. It can show squamous differentiation and stain with HMWK and p63 (nuclear)
Mesenchymal Tumors
♦
Thyroid gland mesenchymal tumors include schwannoma, leiomyoma, solitary fibrous tumor/hemangiopericytoma, malignant peripheral nerve sheath tumor, leiomyosarcoma, and angiosarcoma. Refer to the mesenchymal section of this chapter and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. Most lymphomas involving the thyroid gland are CD20-positive diffuse large B-cell, extranodal marginal-zone B-cell lymphoma of MALT, and rarely follicular lymphoma. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the thyroid gland are kidney, lung, uterus, skin (melanoma), breast, and stomach. Refer to Chapter 3 for further discussion
Parathyroid Gland
♦
Benign parathyroid gland, adenoma, and carcinoma are differentiated by morphology. Immunostains are not useful
Verification Panel
♦
Parathyroid hormone, chromogranin, and pankeratin stain the cytoplasm of parathyroid neoplasms and benign glands
Differential Diagnosis (Table 2.3)
♦
In a gland fragment, thyroid follicular tissue and tumor are in the differential diagnosis. These are positive for TTF–1 (nuclear) and thyroglobulin and negative for parathyroid hormone
Eye and Ocular Adnexa
Malignant Melanoma
Verification Panel
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
HMB 45 and melan-A stain the tumor cell cytoplasm
♦
SOX10 stains melanoma cell nuclei (and “benign” melanocytes, Schwann cells) and is both sensitive and specific for melanocytic lesions
♦
Rare cases show focal pankeratin-positive cytoplasm (3%)
Differential Diagnosis
♦
Carcinoma is diffusely positive for pankeratin; some cases are positive for S-100 (19%) and negative for HMB 45 and melan–A
♦
Small cell carcinoma is positive for pankeratin, chromogranin, synaptophysin, and CD56 and negative for S–100, HMB 45, and melan–A
♦
Schwannoma and malignant peripheral nerve sheath tumors are positive for S-100 and negative for HMB 45 and melan–A
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), Myo–D1 (nuclear); some cases are positive for S-100 (12%) and negative for HMB 45 and melan–A
♦
Retinoblastoma is positive for synaptophysin, chromogranin, NSE, and CD56 and negative for S–100, HMB 45, and melan–A
Retinoblastoma
Verification Panel
♦
Synaptophysin, chromogranin, NSE, CD56, and vimentin stain the tumor cell cytoplasm
♦
GFAP stains the cytoplasm of rare cells that might be entrapped astrocytes or differentiated tumor cells
Differential Diagnosis
♦
Lymphoma is positive for CD45 and CD3 or CD20; some types are positive for CD56 and negative for synaptophysin and chromogranin
♦
Metastatic small cell carcinoma is positive for pankeratin, CD56, chromogranin, and synaptophysin and negative for vimentin
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for CD56, chromogranin, and synaptophysin
♦
Rhabdomyosarcoma is positive for CD56, desmin, myogenin (nuclear), and Myo–D1 (nuclear) and negative for synaptophysin and chromogranin
♦
Ewing sarcoma is positive for CD99 (membranous), Fli–1 (nuclear), and vimentin and negative for CD56, synaptophysin, and chromogranin
Mesenchymal Tumors
♦
Ocular mesenchymal tumors include hemangioma, hemangioblastoma, leiomyoma, inflammatory myofibroblastic tumor, and rhabdomyosarcoma. Refer to the mesenchymal section of this chapter and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. Most lymphomas involving the eye are CD20-positive extranodal marginal-zone B-cell lymphoma of MALT and diffuse large B-cell lymphoma. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
Most lymphomas show CD45 membranocytoplasmic staining. Most lymphomas involving the eye are CD20-positive extranodal marginal-zone B-cell lymphoma of MALT and diffuse large B-cell lymphoma. Refer to the section and chapter on lymphoma for further discussion
Miscellaneous Tumors of the Head and Neck Region
Pleomorphic and Spindle Cell Lipoma
Verification Panel
♦
CD34 stains the tumor cell cytoplasm
♦
S-100 stains the adipocyte nuclei and cytoplasm
Differential Diagnosis
♦
Liposarcoma is positive for S-100 and negative for CD34
Paraganglioma/Carotid Body Tumor
Verification Panel (Fig. 2.6)
♦
Chromogranin and synaptophysin stain the cytoplasm
♦
S-100 stains the nuclei and cytoplasm of the sustentacular cells surrounding the tumor cell nests
♦
Keratin stains the tumor cell cytoplasm focally in some cases (7%)
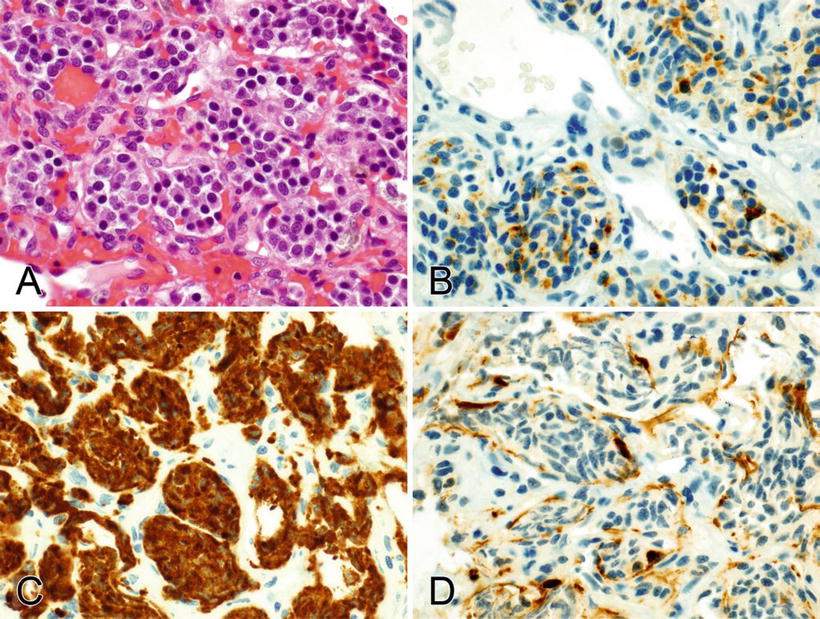
Fig. 2.6.
(A–D) Chromogranin (focally) and synaptophysin (diffusely) stain the cytoplasm in a paraganglioma (carotid body tumor), and S-100 stains the nuclei and cytoplasm of the sustentacular cells surrounding the tumor cell nests.
Differential Diagnosis
♦
Medullary carcinoma of the thyroid is positive for keratin, TTF–1 (nuclear), calcitonin, synaptophysin, and chromogranin
Thorax
Lung
Squamous Cell Carcinoma
♦
IHC may become necessary for the diagnosis of squamous carcinoma when diffusely poor differentiation precludes recognition of keratin production by routine light microscopy
Verification Panel
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm. CK7 focally stains some cases (25%)
♦
p63 and p40 stain the nuclei
♦
Poorly differentiated adenocarcinoma is diffusely positive for CK7 and negative for CK5/6 and p63 (nuclear)
♦
Mesothelioma is positive for CK7, calretinin, D2-40, CK34βe12, CK5/6, and WT1 (nuclear) and negative for p63 and p40 (nuclear)
♦
Metastatic squamous carcinoma to the lung is unusual. IHC does not help identify metastasis except when the lung primary tumor expresses TTF–1 (nuclear)
Table 2.4.
Primary Lung Carcinoma Differential Diagnosis (% Positive Tumors)
Squamous cell carcinoma | Small cell carcinoma | Adenocarcinoma | |
|---|---|---|---|
TTF-1 (nuclear) | 0–15 | 85–100 | 40–85 |
Napsin A | 0–2 | 0 | 70–87 |
p63 | 80–100 | 0–31 | 0–30 |
CK5/6 | 80–100 | 5–25 | 0–20 |
P40 | 100 | 0 | 3 |
Neuroendocrine markersa | 0–10 | 80–100 | 0–10 |
Table 2.5.
Epithelioid Lung and Pleural Tumor Differential Diagnosis (% Positive Tumors)
Calretinin/WT1 a /CK5/6 | CK7 | Additional tumor markers | |
|---|---|---|---|
Mesothelioma | +/+/+ | + | D2-40, WT-1 |
Squamous cell carcinoma | 40/11/+ | 25 | p63a, P40 |
Lung adenocarcinoma | 10/28/5 | + | TTF-1a |
Mucinous lung adenocarcinoma | −/NA/− | + | CDX2a, CK20 |
Metastatic breast carcinoma | 15/−/45 | + | GCDFP-15, mammaglobin |
Metastatic serous ovarian adenocarcinoma | 31/+/22 | + | PAX8, WT-1, MOC-31, Ber-EP4, ERa |
Metastatic renal cell carcinoma | −/8/25 | 11 | RCC Ma, CD10, PAX8b |
Metastatic pancreatobiliary adenocarcinoma | 10/−/20 | + | CA19-9, CK20 |
Metastatic colorectal adenocarcinoma | 18/53/24 | 27 | CDX2a, villin, CK20 |
Metastatic prostatic carcinoma | −/−/− | 10 | PSA, PAP, AMACR |
Metastatic gastric adenocarcinoma | −/11/− | 52 | Shows variable CK7/CK20 pattern |
Metastatic papillary thyroid carcinoma | −/−/14 | + | TTF-1a, thyroglobulin |
Thymic carcinoma | 37/−/+ | 80 | CD5 |
Angiosarcoma, epithelioid hemangioendothelioma | −/29/− | 4 | CD31, CD34, factor VIII, Fli-1a |
Adenocarcinoma
♦
Most pathologists perform IHC on all adenocarcinomas in the lung to confirm the primary site (TTF-1, CK7, and CK20). Refer to Chapter 3 for further discussion
Verification Panel
♦
TTF-1 stains the tumor cell nuclei (85%)
♦
CK7 and CEA stain the tumor cell cytoplasm
♦
ER (nuclear, 61%), CA125 (62%), CA19-9 (38%), HER2 (membranous, 38%), and WT1 (nuclear, 28%) are positive in some cases
♦
CK7, CK20, and CA19-9 stain the cytoplasm and CDX2 the nuclei of mucinous lung adenocarcinomas
♦
GCDFP-15 and p63 (nuclear) are negative in lung adenocarcinoma
♦
CK20 and CDX2 are negative in nonmucinous lung adenocarcinoma
♦
Metastatic carcinoma: TTF-1 (nuclear) is negative in metastatic adenocarcinoma except for rare metastatic thyroid tumors
CK20- positive and CK7-negative carcinomas include colorectal and gastric tumors
CK7- and CK20-positive carcinomas include urothelial, pancreatobiliary, and gastric tumors
CK7- and CK20-negative carcinomas include prostate, clear cell renal cell, and hepatocellular tumors
Breast carcinoma is positive for CK7, GCFDP–15, HER2, and ER/PR (nuclear) and negative for TTF–1 (nuclear) and CK20
Ovarian (nonmucinous) and endometrial adenocarcinomas are positive for CK7 and negative for TTF–1 (nuclear) and CK20
Gastric carcinoma shows variable CK7 and CK20 staining and is negative for TTF–1 (nuclear)
♦
Mesothelioma is positive for CK7, CK5/6, WT1, and calretinin and negative for TTF–1 (nuclear)
♦
Squamous cell carcinoma is positive for CK5/6, p63 (nuclear), and CEA; some cases are positive for CK7 (25%) and TTF-1 (nuclear, 10%)
Pleomorphic, Spindle Cell, Giant Cell, and Sarcomatoid Carcinoma
♦
These undifferentiated tumors show only focal IHC positivity
♦
Melanoma, poorly differentiated sarcomas, and metastatic poorly differentiated carcinomas should be considered. These tumors can show weak or absent antigen reactivity. Additional tumor sampling might demonstrate better differentiated areas
Verification Panel
♦
Pankeratin and vimentin stain the cytoplasm focally
♦
TTF-1 stain scattered nuclei in some cases
Differential Diagnosis
♦
Pulmonary blastoma is positive for pankeratin, β–catenin (nuclear), and vimentin
♦
Mesothelioma is positive for pankeratin, CK7, calretinin, h–caldesmon, D2–40, CK5/6, vimentin, and WT1 (nuclear)
♦
Malignant peripheral nerve sheath tumors (focal) and melanoma (diffuse) are positive for S–100
♦
Leiomyosarcoma is positive for MSA, SMA, and desmin
♦
Synovial sarcoma is positive for bcl–2, CD99 (membranous), pankeratin, EMA, and S–100
♦
Angiosarcoma is positive for CD31, CD34, factor VIII, and Fli–1 (nuclear); some cases are positive for pankeratin (17%) and negative for TTF–1 (nuclear)
♦
Undifferentiated high-grade pleomorphic sarcoma (malignant fibrous histiocytoma) is positive for vimentin
Carcinoid and Atypical Carcinoid Tumors
♦
Neuroendocrine tumors must be graded based on morphology; they show similar IHC patterns
Verification Panel (Fig. 2.7)
♦
Chromogranin, synaptophysin, and CD56 show variable tumor cell cytoplasmic staining
♦
CD56 is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology
♦
Pankeratin stains the tumor cell cytoplasm in most cases (80%)
♦
TTF-1 nuclear staining is variable (30%)
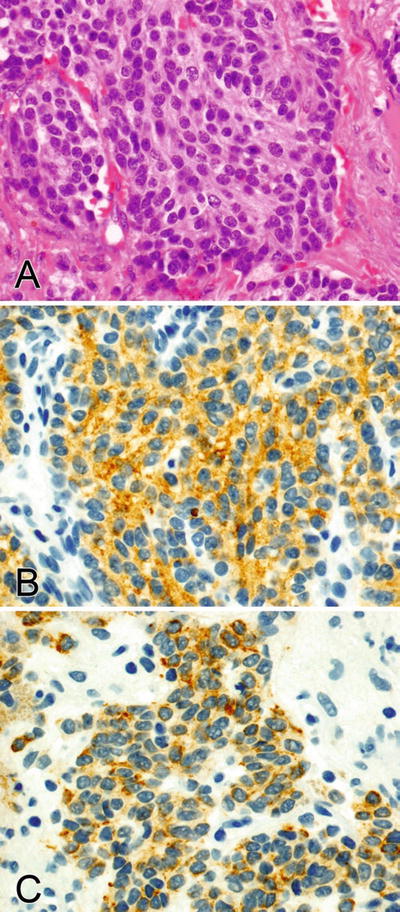
Fig. 2.7.
(A–C) Synaptophysin stains the cytoplasm and pankeratin shows dot-like and diffuse staining in the cytoplasm of a pulmonary carcinoid tumor.
Differential Diagnosis
♦
Paraganglioma is positive for chromogranin and contains S–100-positive spindled sustentacular cells surrounding the tumor cell nests
Small Cell and Large Cell Neuroendocrine Carcinoma
Verification Panel (Fig. 2.8)
♦
Pankeratin and CK7 show dot-like and diffuse cytoplasmic staining
♦
Chromogranin and synaptophysin show variable tumor cell cytoplasmic staining
♦
CD56 is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology
♦
TTF-1 stains the tumor cell nuclei (90% of small cell carcinoma cases). TTF-1 cannot be used to confirm the lung as a primary site in small cell carcinoma; it is positive in many other primary sites including the upper respiratory and gastrointestinal tracts
♦
Calretinin stains some tumors (47%)
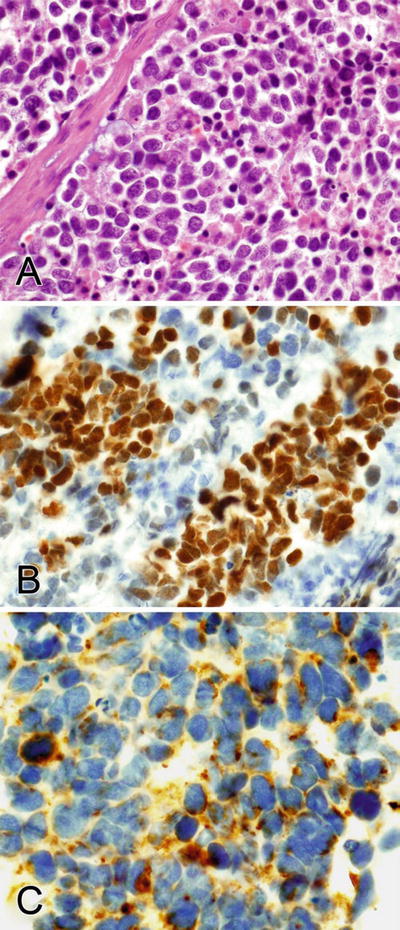
Fig. 2.8.
(A–C) TTF-1 stains the nuclei and pankeratin shows dot-like and diffuse staining in the cytoplasm of a pulmonary small cell carcinoma.
♦
Lymphoma is positive for CD45 and CD3 or CD20 (some types positive for CD56) and negative for pankeratin, chromogranin, synaptophysin, and TTF–1 (nuclear)
♦
Mesothelioma is positive for pankeratin, CK7, calretinin, h-caldesmon, D2–40, CK5/6, and WT1 (nuclear), and some cases are positive for chromogranin (10%), synaptophysin (8%), and CD56 (13%)
♦
Small cell variant of squamous carcinoma is positive for CK5/6 and p63 (nuclear) and negative for chromogranin, synaptophysin, and CD56
♦
Ewing sarcoma is positive for CD99 (membranous) and Fli–1 (nuclear); some cases are positive for pankeratin, synaptophysin, and CD56 and negative for chromogranin
♦
Rhabdomyosarcoma is positive for MSA, desmin, myogenin (nuclear), Myo–D1 (nuclear), and CD56 and negative for pankeratin, chromogranin, and synaptophysin
♦
Melanoma is positive for S-100, HMB 45, and melan–A and negative for pankeratin, chromogranin, synaptophysin, and CD56
Pulmonary Blastoma
♦
This biphasic tumor is composed of malignant glandular and mesenchymal components
Verification Panel
♦
Pankeratin, CEA, and EMA stain the cytoplasm of the epithelial component
♦
Chromogranin, synaptophysin, and various other endocrine markers stain the cytoplasm of the endocrine component
♦
Vimentin, MSA, desmin, and S-100 will stain the mesenchymal, smooth muscle, skeletal muscle, and chondroid components, respectively
♦
β-catenin stains the nuclei and cytoplasm in both the epithelial and stromal components
Differential Diagnosis
♦
Adenocarcinoma does not contain the malignant stromal component
♦
Carcinosarcoma is negative for β–catenin
Sclerosing Hemangioma
Verification Panel
♦
TTF-1 stains the nuclei and EMA the membranes of the round stromal cells and surface cells
♦
Various (CK7, CK20, CAM 5.2) keratins are strongly positive in the cytoplasm of the surface cells and focally positive in the round stromal cells
♦
Vimentin stains the cytoplasm of both cell types
♦
PR stains the nuclei of the round stromal cells
Differential Diagnosis
♦
Adenocarcinoma is positive for CK7; some cases are positive for CK20 (mucinous type) and PR (21%) and negative for vimentin
♦
Carcinoid tumors are positive for pankeratin, chromogranin, synaptophysin, and CD56
♦
Angiosarcoma is positive for CD31, CD34, factor VIII, and Fli–1 (nuclear), and some cases are positive for pankeratin
Clear Cell Tumor (Sugar Tumor)
♦
Part of the perivascular epithelioid cell tumor group (PEComa). These tumors are cytologically bland
Verification Panel
♦
HMB 45, melan-A, SMA, desmin, and vimentin stain the tumor cell cytoplasm
♦
Pankeratin is negative
Differential Diagnosis
♦
Clear cell variant of squamous cell carcinoma is positive for pankeratin, CK5/6, and p63 (nuclear) and negative for HMB 45, melan–A, SMA, and desmin
♦
Adenocarcinoma is positive for pankeratin and TTF–1 (nuclear) and negative for HMB 45, melan–A, SMA, and desmin
♦
Metastatic clear cell renal cell carcinoma is positive for vimentin, RCC Ma, and CD10 and negative for HMB 45, melan–A, SMA, and desmin
♦
Melanoma is positive for S–100, vimentin, HMB 45, and melan-A and negative for SMA and desmin
♦
Granular cell tumor is positive for S–100 and vimentin and negative for HMB 45, melan–A, SMA, and desmin
Bronchial Salivary Gland-Type Neoplasms
♦
The diagnosis of these tumors is based on morphology. These include adenoid cystic carcinoma, mucoepidermoid carcinoma, and epimyoepithelial carcinoma. Refer to the salivary gland section of this chapter
Inflammatory Myofibroblastic Tumor
Verification Panel
♦
ALK is positive in the nuclei and cytoplasm of the tumor cells. This is the most specific marker but stains only 57% of cases
♦
Actin, desmin, and pankeratin focally stain the tumor cell cytoplasm
Differential Diagnosis
♦
Fibrosarcoma is focally positive for SMA and negative for ALK and pankeratin
♦
Leiomyosarcoma is diffusely positive for SMA, MSA, and desmin and negative for ALK
♦
Synovial sarcoma is positive for CD99 (membranous), bcl-2, pankeratin, and EMA; some cases are positive for SMA and negative for ALK
Synovial Sarcoma
♦
IHC cannot differentiate a primary from a metastatic tumor
Verification Panel
♦
CD99, bcl-2, and EMA (77%) stain the tumor cell membranes and cytoplasm
♦
Pankeratin (67%) and SMA (15%) stain the tumor cell cytoplasm
♦
S-100 stains the tumor cell nuclei and cytoplasm in some cases (30%)
Differential Diagnosis
♦
Leiomyosarcoma is diffusely positive for SMA, MSA, and desmin; some cases are positive for CD99 (37%) and bcl-2 (28%) and negative for S–100
♦
Sarcomatoid carcinoma is focally positive for pankeratin, and some cases are positive for CD99, bcl-2, and S-100
♦
Inflammatory myofibroblastic tumor is positive for ALK (57%) and negative for CD99 (membranous)
Other Mesenchymal Tumors
♦
Other pulmonary mesenchymal tumors include chondroma, leiomyoma, schwannoma, solitary fibrous tumor/hemangiopericytoma, epithelioid hemangioendothelioma, primary or metastatic leiomyosarcoma, and angiosarcoma. Metastatic mesenchymal tumors cannot be differentiated from lung primary tumors by IHC or morphology. Refer to the mesenchymal tumor section of this chapter for further discussion
Lymphoma
Verification Panel
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common types are CD20-positive B-cell MALT lymphoma and large cell lymphoma. Refer to the section and chapter on lymphoma for further discussion
Pleura
Mesothelioma
♦
IHC markers are not completely specific or sensitive for mesothelioma, i.e., mesothelioma markers are focally positive in some carcinomas and vice versa. A panel of expected positive and negative markers (usually two of each) provides more definitive results
♦
Calretinin is positive in both epithelioid and sarcomatous types. CK5/6, WT1 (nuclear), and D2-40 are usually negative in sarcomatous mesothelioma
Verification Panel (Fig. 2.9)
♦
Calretinin stains the tumor cell nuclei and cytoplasm
♦
Pankeratin, CK5/6, h-caldesmon, CK7, D2-40, and vimentin stain the tumor cell cytoplasm
♦
WT1 (43–93%) stains the tumor cell nuclei
♦
Thrombomodulin has a membranous pattern
♦
Carcinoma markers are variably (usually focally) positive in mesothelioma: CA125 (48%), CD10 (48%), Ber-EP4 (13%), MOC-31 (8%), RCC Ma (focal, 8%), B72.3 (8%), and LeuMl (5%)
♦
CD99 stains the tumor cell membranes in some cases (53%)
♦
CEA, CA19-9, TTF-1 (nuclear), p63 (nuclear), and ER/PR (nuclear) are negative
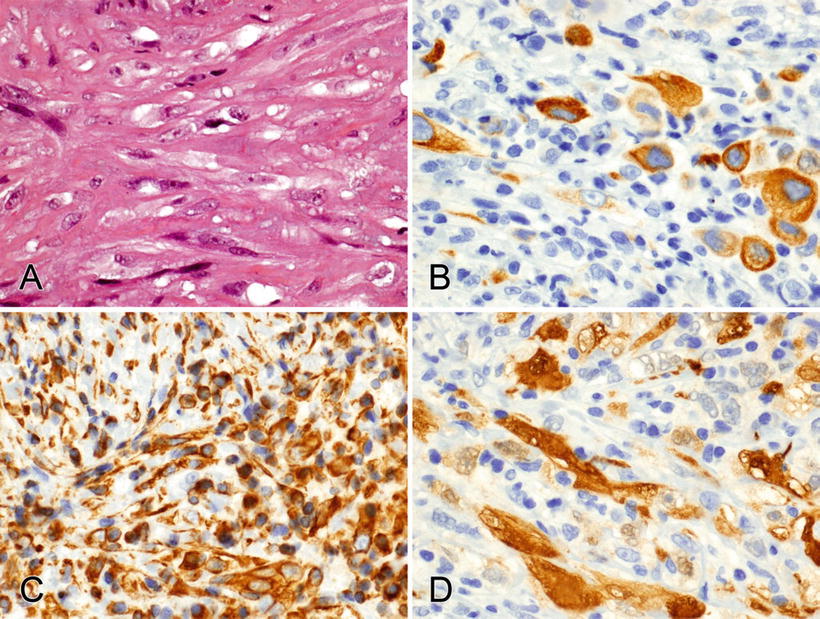
Fig. 2.9.
(A–D) CK7, vimentin, and calretinin stain the cytoplasm of an epithelioid and sarcomatoid mesothelioma.
Differential Diagnosis (Table 2.5)
♦
Lung adenocarcinoma is positive for CK7, MOC-31, TTF–1 (nuclear), napsin A, CEA, Ber–EP4, CA19-9 (68%), and LeuM1 (50%); some cases are positive for calretinin (10%) and CK5/6 (5%) and negative for h–caldesmon, D2–40, WT1 (nuclear), and vimentin
♦
Squamous carcinoma is positive for p63, p40 (nuclear), CK5/6, CEA (77%), and vimentin (60%); some cases are focally positive for calretinin (40%), LeuM1 (30%), Ber-EP4 (40%), and D2-40 (50%) and negative for WT1 (nuclear)
♦
Serous carcinoma is positive for MOC–31, Ber–EP4, ER (nuclear), PAX8, WT1 (nuclear), and C19-9 (67%), and some cases are positive for calretinin (31%), CK5/6 (22%), D2–40 (15%), CEA (13%), and h–caldesmon (5%)
♦
Renal cell carcinoma is positive for RCC Ma, CD10, and LeuM1 (63%); some are positive for MOC-31 (50%), Ber-EP4 (42%), and calretinin (10%) and negative for CEA, B72.3, WT1 (nuclear), and CK5/6
♦
Synovial sarcoma is positive for CD99 (membranous), calretinin, bcl-2, and pankeratin; some cases are positive for CK5/6 (41%), S-100 (21%), and D2-40 (30%) and negative for h–caldesmon and WT1 (nuclear)
♦
Angiosarcoma is positive for CD31, CD34, factor VIII, vimentin, and WT1 (nuclear); some cases are positive for pankeratin (17%) and D2-40 (43%) and negative for calretinin and CK5/6
♦
Melanoma is positive for S–100, HMB 45, and melan–A; some cases are positive for calretinin (20%) and negative for pankeratin
Solitary Fibrous Tumor and Hemangiopericytoma
♦
These tumors are related by IHC and molecular studies
Verification Panel
♦
CD34 stains the tumor cell cytoplasm; endothelial cells are also positive
♦
CD99 and bcl-2 stain the tumor cell membranes and cytoplasm
♦
Desmin, MSA, SMA, and S-100 focally stain some tumors (6–11%)
Differential Diagnosis
♦
Schwannoma is strongly positive for S–100; some are focally positive for EMA (35%) and CD34 (25%)
♦
Leiomyoma is diffusely positive for SMA, MSA, desmin, and bcl-2; some cases are positive for CD99 (27%) and negative for CD34
♦
Synovial sarcoma is positive for CD99, bcl-2, pankeratin, and EMA; some cases are positive for SMA and negative for CD34
Other Mesenchymal Tumors
♦
Other pleural mesenchymal tumors include epithelioid hemangioendothelioma, angiosarcoma, synovial sarcoma, and desmoplastic small round cell tumor. Refer to the mesenchymal tumor section for further discussion
Mediastinum
♦
Thyroid and parathyroid tissues and tumors can occur in the mediastinum. Refer to the head and neck tumor section for further discussion
Thymoma
Thymomas exhibit a mixture of epithelial and lymphoid elements.
Verification Panel
♦
Pankeratin, HMWK (CK34βe12, CK5/6), and CK7 stain the epithelial tumor cell cytoplasm and p63 the nuclei
♦
CD20 stains the cytoplasm of some spindle thymoma cells
♦
CD3, CD1a, CD99, and TdT (nuclear) stain the lymphoid population
Differential Diagnosis
♦
T-lymphoblastic leukemia/lymphoma is positive for CD3, TdT (nuclear), and CD1a and negative for pankeratin, HMWK, and CK7
♦
Hodgkin lymphoma is positive for CD15 and CD30 and negative for CD45, pankeratin, HMWK, and CK7
♦
Germinoma is positive for PLAP and CD117 and negative for pankeratin, HMWK, and CK7
Thymic Carcinoma
♦
The subtype should be identified as squamous, basaloid, mucoepidermoid, etc
Verification Panel (Fig. 2.10)
♦
Pankeratin, CK7, CK19, CAM 5.2, HMWK (CK34βe12, CK 5/6), and CD117 (65%) stain the tumor cell cytoplasm
♦
p63 stains the tumor cell nuclei; PAX8 and TTF1 stain some cases
♦
CD5 stains the tumor cell membranes and cytoplasm (50%)
♦
Calretinin stains the tumor cell nuclei and cytoplasm in some cases (67%)
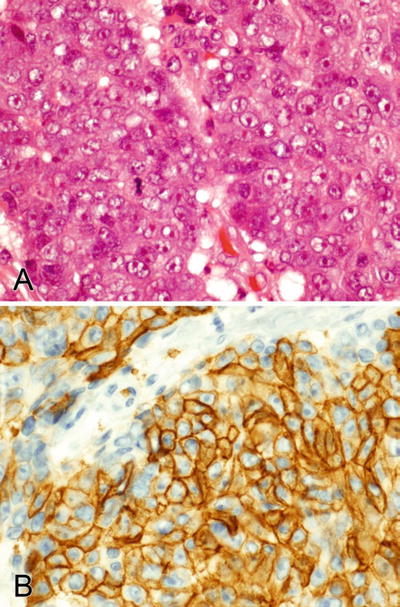
Fig. 2.10.
(A, B) CD5 stains the membranes and cytoplasm of a thymic carcinoma.
Differential Diagnosis
♦
Lung adenocarcinoma is positive for pankeratin, CK7, and TTF–1 (nuclear); some cases are positive for CD5 (61%) and negative for calretinin and p63 (nuclear)
♦
Mesothelioma is positive for calretinin and WT1 (nuclear); some cases are positive for CD5 (69%) and negative for p63 (nuclear)
♦
Germinoma is positive for PLAP, OCT4 (nuclear), and CD117 and negative for keratins and calretinin
♦
Embryonal carcinoma is positive for pankeratin, CD30, and OCT4 (nuclear) and negative for CD5, HMWK, calretinin, and p63 (nuclear)
Thymic Carcinoid and Atypical Carcinoid Tumors
Verification Panel
♦
Chromogranin, synaptophysin, and CD56 show variable tumor cell cytoplasmic staining. CD56 must be evaluated in the context of neuroendocrine morphology
♦
Pankeratin stains the tumor cell cytoplasm
♦
TTF-1 (nuclear) is negative
Differential Diagnosis
♦
Thymoma is positive for pankeratin and negative for CD56, chromogranin, and synaptophysin
Thymic Small Cell Carcinoma
Verification Panel
♦
Pankeratin shows dot-like and diffuse cytoplasmic staining
♦
Chromogranin and synaptophysin show variable tumor cell cytoplasmic staining
♦
CD56 is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology
♦
TTF-1 (nuclear) is negative
Differential Diagnosis
♦
T-lymphoblastic leukemia/lymphoma is positive for CD3, TdT, and CD1a and negative for pankeratin, CD56, chromogranin, and synaptophysin
♦
Hodgkin lymphoma is positive for CD15 and CD30 and negative for CD45, pankeratin, CD56, chromogranin, and synaptophysin
♦
Lung small cell carcinoma is positive for TTF–1 (nuclear)
♦
Ewing sarcoma is positive for CD99 (membranous) and Fli–1 (nuclear); some cases are positive for pankeratin, CD56, and synaptophysin and negative for chromogranin
Germ Cell Tumors
Germinoma
Verification Panel
♦
PLAP and CD117 stain the tumor cell membranes and cytoplasm
♦
OCT4 stains the tumor cell nuclei
♦
Scattered tumor-associated syncytiotrophoblastic giant cells are βhCG and pankeratin positive
♦
CD45 stains the tumor-associated lymphoid cells
♦
Pankeratin is negative or weak and focal (30%)
Differential Diagnosis
♦
Yolk sac tumor is positive for pankeratin, α–FP, and α1–antitrypsin; some cases are focally positive for PLAP (50%) and negative for CD117 and OCT4 (nuclear)
♦
Embryonal carcinoma is positive for OCT4 (nuclear), pankeratin, and CD30 and negative for CD117
♦
Thymic carcinoma is positive for pankeratin; some cases are positive for CD117 (42%) and CD5 (55%) and negative for PLAP
♦
T-lymphoblastic leukemia/lymphoma is positive for CD3, TdT (nuclear), and CD1a and negative for PLAP
♦
Hodgkin lymphoma (classic type) is positive for CD15 and CD30 and negative for CD45 and PLAP
Yolk Sac Tumor (Endodermal Sinus Tumor)
Verification Panel
♦
Pankeratin, α-FP, and α1-antitrypsin stain the tumor cell cytoplasm
Differential Diagnosis
♦
Embryonal carcinoma is positive for pankeratin, OCT4 (nuclear), and CD30 and negative for α-FP and α1–antitrypsin
♦
Thymic carcinoma is positive for pankeratin; some cases are positive for CD117 (42%) and CD5 (55%) and negative for α–FP and α1–antitrypsin
Embryonal Carcinoma
Verification Panel
♦
Pankeratin stains the tumor cell cytoplasm
♦
CD30 and PLAP stain the tumor cell membranes and cytoplasm
♦
OCT4 stains the tumor cell nuclei
♦
Α-FP focally stains some tumors (22%)
Differential Diagnosis
♦
Yolk sac tumor is positive for pankeratin and α-FP and negative for OCT4 (nuclear) and CD30
♦
Germinoma is positive for PLAP, CD117, and OCT4 (nuclear) and negative for CD30 and pankeratin
♦
Thymic carcinoma is positive for pankeratin and HMWK; some cases are positive for CD117 and CD5 and negative for CD30 and PLAP
Choriocarcinoma
Verification Panel
♦
Pankeratin and βhCG stain the tumor cell cytoplasm
Differential Diagnosis
♦
Yolk sac tumors and other carcinomas are βhCG negative
♦
Germinoma is positive for PLAP, OCT4 (nuclear), and CD117 and negative for pankeratin and βhCG (positive in syncytiocytotrophoblasts)
♦
Embryonal carcinoma is positive for pankeratin, βhCG (25%), CD30, and OCT4 (nuclear)
Ewing Sarcoma/Peripheral Neuroectodermal Tumor
Verification Panel
♦
CD99 stains the tumor cell membranes and cytoplasm (cytoplasmic staining alone is a nonspecific finding)
♦
Fli-1 stains the tumor and benign endothelial cell nuclei
♦
Vimentin diffusely stains the tumor cell cytoplasm
♦
Pankeratin (30%), NSE (36%), synaptophysin (9%), and CD56 (9%) focally stain some tumors
Differential Diagnosis
♦
Lymphoblastic lymphoma is positive for CD99 (membranous), CD3, TdT, and CD1a and negative for pankeratin, HMWK, and CK7
♦
Small cell carcinoma is positive for pankeratin, CD56, chromogranin, and synaptophysin and negative for CD99 and Fli–1 (nuclear). Lung tumors are positive for TTF–1 (nuclear)
Other Mesenchymal Tumors
♦
Other mediastinal mesenchymal tumors include lipoma, hemangioma, schwannoma, neurofibroma, paraganglioma, solitary fibrous tumor, synovial sarcoma, neuroblastoma, and malignant peripheral nerve sheath tumor. Refer to the soft tissue tumor section for further discussion
Lymphoma
♦
Mediastinal lymphomas include CD3-positive (CD45 negative) lymphoblastic lymphoma, LeuMl- and CD30-positive (CD45-negative) Hodgkin lymphoma, CD20-positive primary mediastinal B-cell lymphoma, and CD30-positive anaplastic large T-cell lymphoma. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the mediastinum are lung, thyroid, breast, and prostate. Refer to Chapter 3 for further discussion
Heart
Myxoma
Verification Panel
♦
Calretinin (75%), S-100, CD31, CD34, SMA, and MSA show variable staining
♦
Pankeratin is negative
Differential Diagnosis
♦
Metastatic carcinoma is positive for pankeratin
Fibroma
Verification Panel
♦
Vimentin stains the tumor cell cytoplasm
Differential Diagnosis
♦
Leiomyoma is positive for SMA, MSA, and desmin
Rhabdomyoma and Rhabdomyosarcoma
Verification Panel
♦
Desmin and MSA stain the tumor cell cytoplasm
♦
Myogenin and Myo-D1 stain the tumor cell nuclei
Differential Diagnosis
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Carcinoma is positive for pankeratin and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for MSA, desmin, myogenin (nuclear), and Myo–D1 (nuclear)
Hemangioma, Epithelioid Hemangioendothelioma, and Angiosarcoma
Verification Panel
♦
CD31, CD34, and factor VIII stain the tumor cell cytoplasm; benign endothelial cells are also positive
♦
Fli-1 stains the tumor and benign endothelial cell nuclei
♦
Pankeratin can be focally positive in angiosarcoma (17%)
Differential Diagnosis (Table 2.5)
♦
Poorly differentiated carcinoma is diffusely positive for pankeratin and negative for CD31, CD34, factor VIII, and Fli–1 (nuclear)
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for pankeratin, CD31, CD34, factor VIII, and Fli–1 (nuclear)
Mesothelioma
♦
These cardiac tumors show the same IHC pattern and differential diagnosis as the pleural tumors (see discussion above)
Other Mesenchymal Tumors
♦
Other cardiac mesenchymal tumors include lipoma, inflammatory myofibroblastic tumor, leiomyosarcoma, synovial sarcoma, solitary fibrous tumor, and undifferentiated high-grade pleomorphic sarcoma. Refer to the soft tissue tumor section for further discussion
Lymphoma
♦
Systemic disease commonly involves the heart and most show CD45 membranocytoplasmic staining. Rare primary lymphomas are CD20-positive large B-cell type. Refer to the lymphoma section for further discussion
Digestive Tract
Esophagus
Infection
♦
IHC antibodies to herpes simplex virus (HSV1 and HSV2) show nuclear and cytoplasmic staining of infected squamous cells
♦
IHC antibody to cytomegalovirus shows nuclear and occasionally cytoplasmic staining of infected cells (endothelial, stromal, and occasionally glandular)
Squamous Cell Carcinoma
♦
IHC may become necessary for the diagnosis of squamous carcinoma when diffusely poor differentiation precludes recognition of keratin production by routine light microscopy
Verification Panel
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm
♦
p63 and p40 stain the tumor cell nuclei
Differential Diagnosis
♦
Poorly differentiated adenocarcinoma is positive for CEA and negative for CK5/6 and p63 (nuclear)
♦
Direct extension from a lung primary tumor can be identified when it is positive for TTF–1 (nuclear, 23%)
High-Grade Dysplasia in Barrett Esophagus
♦
Diagnosis is based on morphologic assessment; IHC may be helpful in limited biopsies
Verification Panel (Fig. 2.11)
♦
p53 stains the dysplastic cell nuclei (70%)
♦
Ki-67 proliferation of the dysplastic cell nuclei extends to the surface epithelium
♦
Cyclin D1 nuclear expression is increased (45%)
Fig. 2.11.
(A) Ki-67 shows increased proliferation in the central area of the glands of low-grade dysplasia associated with Barrett esophagus. (B) Ki-67 shows proliferation extending into the surface in high-grade dysplasia. (C) p53 expression in the nuclei of high-grade dysplasia.
Differential Diagnosis
♦
Low-grade dysplasia and reactive/regenerative changes are negative for p53 (nuclear) and cyclin D1 (nuclear) and do not show Ki-67 (nuclear) proliferation in the surface epithelium
Adenocarcinoma
Verification Panel
♦
Pankeratin stains the tumor cell cytoplasm
♦
CDX2 stains the tumor cell nuclei
♦
No specific CK7/CK20 pattern is useful for this site
Differential Diagnosis
♦
Direct extension of a lung primary tumor is usually positive for TTF–1 (nuclear) and napsin A and negative for CDX2 (nuclear)
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for pankeratin
Small Cell Carcinoma
Verification Panel
♦
Pankeratin shows dot-like or diffuse cytoplasmic staining
♦
Chromogranin and synaptophysin show variable tumor cell cytoplasmic staining
♦
CD56 (membranocytoplasmic) is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology and keratin staining
♦
TTF-1 stains the tumor cell nuclei (71%)
Differential Diagnosis
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for pankeratin
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for pankeratin
Malignant Melanoma
Verification Panel
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
HMB 45 and melan-A stain the tumor cell cytoplasm
♦
Rare cases show focal pankeratin-positive cytoplasm (3%)
Differential Diagnosis
♦
Carcinoma is diffusely positive for pankeratin; some cases are focally positive for S-100 (19%) and negative for HMB 45, and melan–A
♦
Small cell carcinoma is positive for pankeratin, chromogranin, synaptophysin, and CD56 and negative for HMB 45 and melan–A
Leiomyoma/Leiomyosarcoma
Verification Panel
♦
SMA, MSA, calponin, caldesmon, and desmin stain the tumor cell cytoplasm
♦
Some tumors are focally positive for pankeratin (40%)
Differential Diagnosis (Table 2.6)
♦
Gastrointestinal tumor is positive for CD117, DOG1, and CD34 and can be focally positive for SMA
Table 2.6.
Gastrointestinal Spindle Cell Tumor Differential Diagnosis a (% Positive Tumors)
CD117 | DOG1 | CD34 | ALK-1 | SMA, MSA and desmin | S-100 | β-catenin (nuclear) | |
|---|---|---|---|---|---|---|---|
GIST | 95 | 95 | 70 | NA | 48 | − | 5 |
IMFT | 5 | NA | 6 | 57 | + | − | − |
Leiomyoma/leiomyosarcoma | 7 | 0 | 7 | − | + | 6 | − |
Schwannoma | − | 10 | − | 18 | − | + | − |
Fibromatosis | 30 | NA | − | − | 81 | − | 92 |
Granular Cell Tumor
Verification Panel
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
Inhibin and CD68 stain the tumor cell cytoplasm
♦
Epithelial markers (various keratins and EMA) are negative
Differential Diagnosis
♦
Adenocarcinoma is positive for pankeratin and negative for S–100 and inhibin
♦
Histiocytic inflammation is positive for S-100 and CD68 and negative for inhibin
Other Mesenchymal Tumors
♦
Other esophageal mesenchymal tumors include gastrointestinal stromal tumor, rhabdomyosarcoma, and Kaposi sarcoma. Refer to the mesenchymal section and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. Most lymphomas involving the esophagus are secondary from the mediastinum, nodes, and stomach. The most common primaries are CD20-positive diffuse large B-cell lymphoma and extranodal marginal-zone B-cell lymphoma of MALT. Refer to the lymphoma section and chapter for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the esophagus are thyroid, lung, breast, skin (melanoma), kidney, prostate, and ovary. Refer to Chapter 3 for further discussion
Stomach
Adenocarcinoma
Verification Panel
♦
Pankeratin stains the tumor cell cytoplasm
♦
CDX2 stains the tumor cell nuclei (73%)
♦
No specific CK7/CK20 pattern is useful for this site
Differential Diagnosis
♦
Metastatic breast lobular adenocarcinoma is positive for ER/PR (nuclear, 89%), mammaglobin, and GCDFP–15 and negative for CK20 and CDX2 (nuclear)
♦
Epithelioid gastrointestinal stromal tumor is positive for CD117 and CD34 and negative for pankeratin
Hepatoid Adenocarcinoma
Verification Panel
♦
Pankeratin, HepPar1, and alpha-fetoprotein stain the tumor cell cytoplasm focally
Differential Diagnosis
♦
Metastatic hepatocellular adenocarcinoma cannot be differentiated by IHC
Well-Differentiated Endocrine Neoplasms
Verification Panel
♦
Pankeratin stains the tumor cell cytoplasm (sometimes weakly)
♦
Chromogranin and synaptophysin show variable tumor cell cytoplasmic staining
♦
CD56 (membranocytoplasmic) is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology and keratin staining
Differential Diagnosis
♦
Glomus tumor is positive for SMA and negative for pankeratin, chromogranin, synaptophysin, and CD56
♦
Well-differentiated adenocarcinoma is positive for pankeratin and negative for chromogranin, synaptophysin, and CD56
Gastrointestinal Stromal Tumor (GIST)
Verification Panel (Fig. 2.12)
♦
CD117 (95%) and CD34 (70%) stain the tumor cell membranes and cytoplasm; CD34 also stains endothelial cells
♦
SMA can be focally positive (25%)
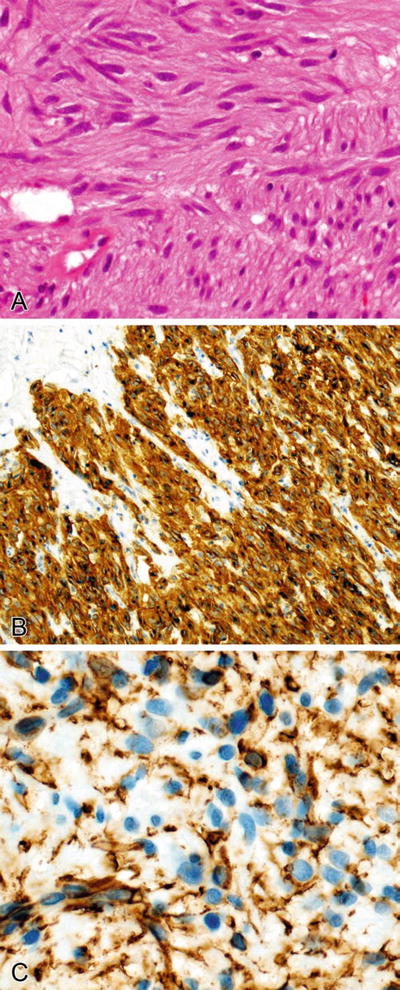
Fig. 2.12.
(A–C) CD117 and CD34 stain the cytoplasm and membranes of a gastrointestinal stromal tumor. CD34 also stains the endothelial cells of a benign small vessel (lower left).
Differential Diagnosis (Table 2.6)
♦
Leiomyoma and leiomyosarcoma are diffusely positive for SMA, MSA, calponin, caldesmon and desmin and negative for CD117 and CD34
♦
Schwannoma (diffusely) and malignant peripheral nerve sheath tumor (focally) are positive for S-100 and negative for CD117 and CD34
♦
Fibromatosis is positive for SMA (focally), β–catenin (nuclear), and CD117 (30%) and negative for CD34
♦
Poorly differentiated carcinoma (vs. epithelioid GIST ) is positive for pankeratin and negative for CD117 and CD34
Glomus Tumor
Verification Panel
♦
SMA diffusely stains the tumor cell cytoplasm
♦
CD34 focally stains the tumor cell membranes and cytoplasm (53%)
Differential Diagnosis
♦
Carcinoid tumor is positive for pankeratin, chromogranin, synaptophysin, and CD56 and negative for SMA and CD34
♦
Epithelioid gastrointestinal tumor is positive for CD117 and CD34 and can be focally positive for SMA
Other Mesenchymal Tumors
♦
Other gastric mesenchymal tumors include gastrointestinal autonomic nerve tumor, schwannoma, lipoma, granular cell tumor, leiomyoma, leiomyosarcoma, rhabdomyosarcoma, and Kaposi sarcoma. Refer to the mesenchymal section and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common primaries are CD20-positive diffuse large B-cell lymphoma and extranodal marginal-zone B-cell lymphoma of MALT. Refer to the lymphoma section and chapter for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the stomach are breast, skin (melanoma), lung, and other gastrointestinal carcinomas. Refer to Chapter 3 for further discussion
Small Intestine, Colon, and Rectum
Infection (Fig. 2.13)
Fig. 2.13.
(A, B) CMV stains the nuclei and occasionally the cytoplasm of infected cells in cytomegalovirus colitis.
♦
IHC antibody to cytomegalovirus shows nuclear and occasionally cytoplasmic staining of infected cells (endothelial, stromal, and occasionally glandular)
Adenocarcinoma
♦
CDX2 (nuclear) stains most colorectal adenocarcinomas (90–100%) and many small intestinal adenocarcinomas (65%)
♦
Villin (membranocytoplasmic) stains most colorectal adenocarcinomas (90–100%) and many small intestinal adenocarcinomas (71%)
♦
Microsatellite instability IHC can be performed when clinically appropriate for the evaluation of hereditary nonpolyposis colon cancer syndrome. MLH1, MSH2, MSH6, and/or PMS2 nuclear staining is lost when abnormal (Fig. 2.14)

Fig. 2.14.
PMS2 shows abnormal loss of nuclear staining in a colon cancer (upper left) indicating a loss of mismatch repair genes associated with nonpolyposis hereditary colon cancer. A fragment of benign mucinous colonic epithelium shows normal nuclear staining (lower right).
Verification Panel
♦
Small intestinal adenocarcinoma expresses CK7 with variable strength and is frequently CK20 positive (56%) (normal small intestinal mucosa is CK20 positive and CK7 negative); AMACR (5%) is negative
♦
Colorectal adenocarcinoma is CK20 positive (94%), usually AMACR positive (82%), and occasionally CK7 positive (10%)
Differential Diagnosis
♦
Colorectal adenocarcinoma directly invading into or metastatic to the small bowel is positive for CD20 and AMACR and negative for CK7
♦
Epithelioid gastrointestinal tumor is positive for CD117 and CD34 and negative for pankeratin
Well-Differentiated Endocrine Neoplasms
Verification Panel
♦
Pankeratin stains the tumor cell cytoplasm (often weakly)
♦
Chromogranin and synaptophysin show variable tumor cell cytoplasmic staining
♦
CD56 (membranocytoplasmic) is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology and keratin staining
Differential Diagnosis
♦
Well-differentiated adenocarcinoma is positive for pankeratin and negative for chromogranin, synaptophysin, and CD56
Small Cell Neuroendocrine Carcinoma
Verification Panel
♦
Pankeratin shows punctuate or diffuse tumor cell cytoplasmic staining
♦
Chromogranin and synaptophysin show variable tumor cell cytoplasmic staining
♦
CD56 (membranocytoplasmic) is more sensitive for neuroendocrine differentiation but less specific; it must be evaluated in the context of neuroendocrine morphology and keratin staining
Differential Diagnosis
♦
Lymphoma is positive for CD45 and CD3 or CD20 and negative for keratin
♦
Melanoma is positive for S-100, HMB 45, and melan–A and negative for pankeratin
Granular Cell Tumor
Verification Panel
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
Inhibin and CD68 stain the tumor cell cytoplasm
♦
Epithelial markers (various keratins and EMA) are negative
Differential Diagnosis
♦
Adenocarcinoma is positive for pankeratin; some cases are positive for S-100 and negative for CD68 and inhibin
♦
Histiocytic inflammation is positive for CD68 and S-100 and negative for inhibin
Gastrointestinal Stromal Tumor (GIST)
Verification Panel
♦
CD117 (95%), DOG1 (95%), and CD34 (70%) stain the tumor cell membranes and cytoplasm; CD34 also stains endothelial cells
♦
SMA (25%) can be focally positive
Differential Diagnosis (Table 2.6)
♦
Leiomyoma and leiomyosarcoma are diffusely positive for SMA, MSA, calponin, and desmin and negative for CD117 and CD34
♦
Schwannoma (diffusely) and malignant peripheral nerve sheath tumor (focally) are positive for S-100 and negative for CD117 and CD34
♦
Fibromatosis is positive for SMA (focally) and β-catenin (nuclear); some are positive for CD117 (30%) and negative for CD34
♦
Poorly differentiated carcinoma (vs. epithelioid GIST) is positive for pankeratin and negative for CD117 and CD34
Fibromatosis
Verification Panel
♦
β-catenin stains the tumor cell nuclei
♦
CD117 stains some tumors (30%)
♦
SMA, MSA, and desmin stain the tumor cell cytoplasm focally
Differential Diagnosis (Table 2.6)
♦
Leiomyoma and leiomyosarcoma are diffusely positive for SMA, MSA, and desmin and negative for β–catenin (nuclear) and CD117
♦
Inflammatory myofibroblastic tumor is positive for SMA, MSA, desmin, and ALK (57%) and negative for β–catenin (nuclear) and CD117
♦
Gastrointestinal stromal tumor is positive for CD117, DOG1, CD34, and SMA and negative for β–catenin (nuclear)
Inflammatory Myofibroblastic Tumor
Verification Panel
♦
ALK stains the tumor cell nuclei. This marker is the most specific for this tumor but stains only 57% of cases
♦
SMA, MSA, desmin, and keratin (30%) stain the tumor cell cytoplasm focally
Differential Diagnosis (Table 2.6)
♦
Fibromatosis is positive for β–catenin (nuclear), focally positive for SMA and CD117, and negative for ALK
♦
Fibrosarcoma is negative for ALK, SMA, MSA, and desmin
♦
Gastrointestinal stromal tumor is positive for CD117, CD34, and SMA and negative for ALK
Other Mesenchymal Tumors
♦
Other small intestinal and colorectal mesenchymal tumors include lymphangioma, neurofibroma, ganglioneuroma, leiomyoma, lipoma, Kaposi sarcoma, leiomyosarcoma, and angiosarcoma. Refer to the mesenchymal section and Chapter 3 for further discussion
Lymphoma
Verification Panel
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common types in the small intestine and colon are CD20-positive B-cell MALT, mantle cell, Burkitt, and diffuse large cell lymphoma. Small intestinal enteropathy-related T-cell lymphoma is CD45 and CD3 positive. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the small intestine and colon are skin (melanoma), lung, breast, kidney, and other gastrointestinal carcinomas. Refer to Chapter 3 for further discussion
Anus
Infection
♦
IHC antibody to herpes simplex virus (HSV1 and HSV2) shows nuclear and cytoplasmic staining of infected squamous cells
High-Grade Squamous Dysplasia/Carcinoma In Situ/Bowen Disease
Verification Panel
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm
♦
p63 stains the tumor cell nuclei
♦
Ki-67 shows an increased proliferation rate into the upper one-third of the epithelium
♦
p16 stains the tumor cell nuclei and cytoplasm in HPV-related lesions
Differential Diagnosis
♦
Melanoma in situ is positive for S–100, HMB 45, and melan–A and negative for keratin
♦
Extramammary Paget disease is positive for CEA, GCDFP–15, and HER2; some cases are positive for S-100 and negative for HMWK, HMB 45, and melan–A
♦
Atrophy is negative for p16 and shows Ki–67 (nuclear) proliferation limited to the basal layers
Squamous Cell Carcinoma
♦
IHC may become necessary for the diagnosis of squamous carcinoma when diffusely poor differentiation precludes recognition of keratin production by routine light microscopy
Verification Panel
♦
HMWK (CK34βe12, CK5/6) stains the tumor cell cytoplasm
♦
p63 stains the tumor cell nuclei
♦
p16 stains the tumor cell nuclei and cytoplasm in HPV-related lesions
Differential Diagnosis
♦
Poorly differentiated adenocarcinoma is strongly positive for CEA and negative for CK5/6 and p63
♦
Melanoma is positive for S–100, HMB 45, and melan–A and negative for pankeratin
♦
Basal cell carcinoma is positive for Ber–EP4 and negative for CEA and EMA
Extramammary Paget Disease
♦
Two types have been demonstrated by IHC testing: primary and secondary to underlying malignancy
Verification Panel
♦
CK7- and GCDFP-15-positive and CK20-negative tumors are not associated with colorectal or other carcinomas (primary extramammary Paget disease) and possibly derive from the anal sweat glands
♦
CK20-positive, occasionally CK7-positive (13%), and GCDFP-15-negative tumors are often associated with colorectal, urothelial, or prostatic adenocarcinoma (probable pagetoid spread into the epidermis)
Differential Diagnosis
♦
Melanoma in situ is positive for S-100, HMB 45, and melan–A and negative for pankeratin, CK7, CK20, and GCDFP–15
♦
Bowen disease is positive for CK5/6, focally positive for CK7, and negative for CK20 and GCDFP–15
Anal Gland Adenocarcinoma
Verification Panel
♦
CK7 stains the tumor cell cytoplasm
♦
CDX2 (nuclear) is negative
Differential Diagnosis
♦
Rectal adenocarcinoma is positive for CK20 and CDX2 (nuclear) and negative for CK7
Malignant Melanoma
Verification Panel
♦
S-100 stains the tumor cell nuclei and cytoplasm
♦
HMB 45 and melan-A stain the tumor cell cytoplasm
♦
Rare cases show focal pankeratin-positive cytoplasm (3%)
Differential Diagnosis
♦
Carcinoma is strongly positive for pankeratin; some cases are positive for S-100 and negative for HMB 45 and melan–A
♦
Small cell carcinoma is positive for pankeratin and chromogranin, synaptophysin, and CD56 and negative for HMB 45 and melan–A
Leiomyoma/Leiomyosarcoma
Verification Panel
♦
SMA, MSA, and desmin stain the tumor cell cytoplasm
♦
Some tumors are focally positive for pankeratin (40%)
Differential Diagnosis (Table 2.6)
♦
Gastrointestinal stromal tumor is positive for CD117 and CD34 and can be focally positive for SMA
Other Mesenchymal Tumors
♦
Other anal mesenchymal tumors include hemangioma, lymphangioma, leiomyoma, schwannoma, neurofibroma, lipoma, hemangiopericytoma, aggressive angiomyxoma, rhabdomyosarcoma, fibrosarcoma, and Kaposi sarcoma. Refer to the mesenchymal section and Chapter 3 for further discussion
Lymphoma
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common primary tumor is CD20-positive large B-cell lymphoma. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the anus are colorectal, lung, breast, and pancreatic. Refer to Chapter 3 for further discussion
Hepatobiliary
Liver
♦
Ubiquitin and pankeratin stain Mallory bodies
♦
α1-antitrypsin stains the globules in livers with this enzyme deficiency
Infection
♦
IHC antibodies to hepatitis B show nuclear staining for HBV core and cytoplasmic staining for HBV surface (Fig. 2.15)

Fig. 2.15.
HBV surface (A) stains the cytoplasm and HBV core (B) stains the nuclei in a hepatitis B infected liver.
♦
IHC antibodies to herpes simplex virus (HSV1 and HSV2) show nuclear and cytoplasmic staining of infected cells
♦
IHC antibody to cytomegalovirus shows nuclear and occasionally cytoplasmic staining of infected cells (endothelial, stromal, and epithelial)
Hepatic Adenoma
Verification Panel
♦
HepPar1 stains the tumor cell cytoplasm; benign liver cells are also positive
♦
CD10 and polyclonal CEA stain the bile canaliculi
♦
CD34 is negative or patchy showing lack of capillarization
Differential Diagnosis
♦
Hepatocellular carcinoma shows diffuse CD34-positive neocapillarization around tumor nests and cords
Hepatocellular Carcinoma
Verification Panel (Fig. 2.16)
♦
HepPar1 and α-FP (25%) stain the tumor cell cytoplasm. HepPar1 also stains benign liver cells
♦
CD10 and polyclonal CEA stain the bile canaliculi
♦
CD34 stains the neocapillarization around tumor cell nests and cords
♦
Pankeratin (10%), CK7 (24%), and CK20 (7%) are usually focal or negative
♦
Arginase-1 is promising as a sensitive and specific marker for hepatocellular carcinoma and glypican-3 to a lesser extent
♦
A panel that incorporates HepPar1, arginase-1 and glypican-3 may be of great value in the differential diagnosis of hepatocellular carcinoma versus cholangiocarcinoma and/or metastatic adenocarcinoma
Differential Diagnosis (Table 2.7)
♦
Hepatic adenoma does not show the CD34-positive neocapillarization pattern
♦
Cholangiocarcinoma is positive for CK7, CK20, and CA19-9; does not have the CD10 and pCEA bile canalicular pattern staining; and is negative for HepPar1
♦
Metastatic carcinoma is often positive for CK7 or CK 20; does not have the CD10 and pCEA bile canalicular pattern staining; and is negative for HepPar1. See Table 2.7 and the unknown chapter for further discussion
♦
Angiosarcoma is positive for CD31, CD34, factor VIII, and Fli–1 (nuclear); some cases are positive for pankeratin (17%) and CD10 and negative for HepPar1
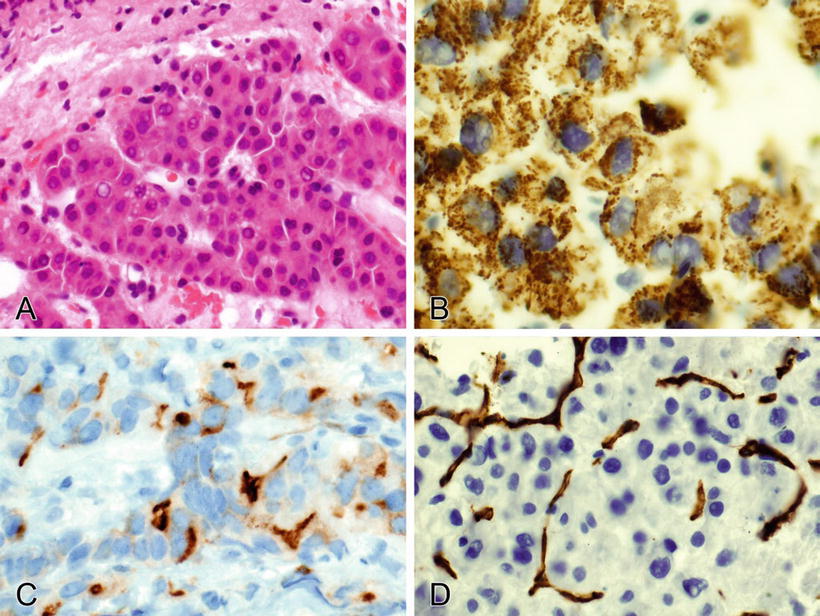
Fig. 2.16.
(A–D) HepPar1 stains the tumor cell cytoplasm, CD10 stains the bile canaliculi, and CD34 highlights the neocapillarization in a hepatocellular carcinoma.
Table 2.7.
Hepatic Tumor Differential Diagnosis (% Positive Tumors)
HepPar-1, pCEA a , CD10 a | CK7 | CK20 | CA19-9 | CD31, CD34, Fli-1 (nuclear) b | CD45 | |
|---|---|---|---|---|---|---|
Hepatocellular carcinoma | + | 24 | 7 | − | − | − |
Cholangiocarcinoma | − | 97 | 23 | + | − | − |
Metastatic carcinoma | 0–15/−/− | Variesc | Varies | Varies | − | − |
Angiosarcoma | − | 25 | − | − | + | − |
Lymphoma | − | − | − | − | − | + |
Hepatoblastoma
Verification Panel
♦
HepPar1 stains the tumor cell cytoplasm
♦
α-FP (58%) and pankeratin (84%) stain many tumors
♦
CD99 stains the membranes (diagnostic) and cytoplasm of most tumors (75%)
♦
CD56 (33%), synaptophysin, and chromogranin stain some tumors focally
Differential Diagnosis
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), and Myo–D1 (nuclear) and negative for HepPar1 and α–FP
♦
Ewing sarcoma is positive for CD99 (membranous) and Fli–1 (nuclear) and negative for HepPar1 and α–FP
♦
Neuroblastoma is positive for CD99, synaptophysin, chromogranin, and CD56 and negative for HepPar1
♦
Wilms tumor is positive for WT1 (nuclear) and negative for HepPar1
Cholangiocarcinoma
Verification Panel
♦
CK7, CK20 (23%), CA19-9 (84%), and monoclonal CEA stain the tumor cell cytoplasm and CDX2 (21%) stains the tumor cell nuclei
Differential Diagnosis (Table 2.7)
♦
Hepatocellular carcinoma is positive for HepPar1; some cases are positive for CK7 (24%) and negative for CK20, mCEA, and CA19–9
♦
♦
Cholangiocarcinoma diagnosis usually rests on a combination of morphology and IHC to exclude hepatocellular carcinoma and metastatic adenocarcinoma, as there is currently no established primary marker for cholangiocarcinoma in general use
Hemangioma, Infantile Hemangioendothelioma, Epithelioid Hemangioendothelioma, and Angiosarcoma
♦
These tumors are differentiated based on morphology; the IHC staining pattern is the same
Verification Panel
♦
CD31, CD34, and factor VIII stain the tumor benign endothelial cells, which are also positive
♦
Fli-1 stains the tumor and benign endothelial cell nuclei
♦
Pankeratin can be focally positive in the tumor cell cytoplasm (17%)
Differential Diagnosis (Table 2.7)
♦
Hepatocellular carcinoma is positive for HepPar1, and the tumor cells are negative for CD31, CD34, factor VIII, and Fli–1 (nuclear); however, these demonstrate neocapillarization
♦
Poorly differentiated metastatic carcinoma is diffusely positive for pankeratin and negative for CD31, CD34, factor VIII, and Fli–1 (nuclear)
Undifferentiated Sarcoma
♦
IHC is not diagnostic but is useful in the differential diagnosis. PAS with diastase highlights the distinctive tumor globules
Verification Panel
♦
Vimentin stains diffusely and keratin focally in the tumor cell cytoplasm
Differential Diagnosis
♦
Rhabdomyosarcoma is positive for desmin, myogenin (nuclear), Myo–D1 (nuclear), and vimentin
♦
Hepatoblastoma is positive for HepPar1, α–FP, and vimentin
Embryonal Rhabdomyosarcoma
Verification Panel
♦
Desmin and MSA stain the tumor cell cytoplasm
♦
Myogenin and Myo-D1 stain the tumor cell nuclei
Differential Diagnosis
♦
Hepatoblastoma is positive for HepPar1 and α-FP and negative for desmin, MSA, myogenin (nuclear), and Myo–D1 (nuclear)
♦
Undifferentiated sarcoma is negative for desmin, MSA, Myo–D1 (nuclear), and myogenin (nuclear)
Angiomyolipoma
Verification Panel
♦
HMB 45, melan-A, SMA, MSA, and desmin are positive in the cytoplasm of the spindle and epithelioid tumor cells
♦
Keratins, EMA, and S-100 are negative (S-100 stains the adipose elements)
Differential Diagnosis
♦
Adipose tumors (e.g., liposarcoma) are positive for S-100 and negative for HMB 45, melan–A, SMA, MSA, and desmin
♦
Leiomyoma and leiomyosarcoma are diffusely positive for MSA, SMA, and desmin and negative for HMB 45 and melan–A
Other Mesenchymal Tumors
♦
Other hepatic mesenchymal tumors include lymphangioma, solitary fibrous tumor/hemangiopericytoma, lipoma, inflammatory myofibroblastic tumor, and Kaposi sarcoma. Refer to the mesenchymal section and Chapter 3 for further discussion
Lymphoma (Table 2.7)
♦
Most lymphomas show CD45 membranocytoplasmic staining. The most common type is CD20-positive diffuse large B-cell lymphoma. Less frequent are Burkitt lymphoma and extranodal marginal-zone B-cell lymphoma of MALT. Refer to the section and chapter on lymphoma for further discussion
Metastatic Disease
♦
The most common tumors to metastasize to the liver are colon, stomach, gallbladder, pancreas, lung, breast, esophagus, and genitourinary. Knowledge of clinical history, imaging studies, serum tumor markers, and thorough morphologic assessment can significantly reduce the extent of the panels needed to identify these tumors. Refer to Chapter 3 for further discussion
Pancreas
Invasive Ductal Carcinoma
♦
IHC is generally unnecessary for the diagnosis of ductal carcinoma except when poorly differentiated
♦
Reactive changes must be differentiated by morphology, clinical history, and imaging features; IHC is not diagnostic per se
Verification Panel
♦
CK7 (94%) diffusely and CK20 (52%) focally stain the tumor cell cytoplasm
♦
CA19-9 and mCEA stain the tumor cell membranes and cytoplasm
Differential Diagnosis
♦
Ampullary adenocarcinoma is positive for MUC2, more diffusely positive for CDX2 (nuclear), and negative for MUC1 and CK17; pancreatic ductal carcinoma is positive for MUC1 and CK17, heterogeneous for CDX2 (nuclear), and negative for MUC2
♦
Acinar cell carcinoma is positive for pankeratin, α1–antitrypsin, lipase, trypsin, and chymotrypsin and negative for CK7, CA19–9, and mCEA
♦
Neuroendocrine tumor is positive for pankeratin, chromogranin, synaptophysin, and CD56 and negative for CA19–9
Mucinous Cystic Neoplasms
♦
Tumor location (tail, body), tumor unconnected to the ductal system, and female gender are characteristics of this tumor
♦
The glandular elements show the same IHC profile as invasive ductal adenocarcinoma (see above). The associated ovarian-type stroma shows distinctive IHC features
Verification Panel
♦
Progesterone receptor (PR) stains the nuclei of the ovarian-type stroma
♦
Smooth muscle actin (SMA), muscle-specific actin (MSA), desmin, inhibin, and calretinin stain the cytoplasm of the ovarian-type stroma
Differential Diagnosis
♦
Invasive ductal carcinoma lacks the PR (nuclear) and SMA/MSA/desmin-positive ovarian-type stroma
♦
Intraductal papillary mucinous neoplasms lack the PR (nuclear) and SMA/MSA/desmin-positive ovarian-type stroma
Intraductal Papillary Mucinous Neoplasms
♦
Multiple morphological subtypes with corresponding IHC profiles have been identified. IHC is usually unnecessary
Verification Panel
♦
The intestinal type is positive for CDX2 (nuclear) and MUC1 and negative for MUC2 (8%) and is most frequently associated with colloid carcinoma
♦
The pancreatobiliary type is more frequently positive for MUC2 (44%) and negative for CDX2 (nuclear) and MUC1 and is associated with the typical pancreatic ductal adenocarcinoma
Differential Diagnosis
♦
Invasive ductal carcinoma must be identified by the lack of a predominantly cystic papillary mucinous component
♦
Mucinous cystic neoplasms have a distinct epidemiology and contain ovarian-type stroma that is positive for PR (nuclear), SMA, MSA, and desmin
Serous Cystic Neoplasms
♦
Diagnosis is based on morphology; IHC is usually unnecessary
Verification Panel
♦
CK7 diffusely and CA19-9 and B72.3 focally stain the tumor cell cytoplasm
♦
mCEA is negative
Differential Diagnosis
♦
Ductal carcinoma is positive for mCEA, CA19-9, B72.3, CK7, and CK20 (52%)
Acinar Cell Carcinoma
♦
PAS–diastase special stain demonstrates the cytoplasmic zymogen granules
Verification Panel
♦
Pankeratin, α1-antitrypsin, lipase, trypsin, and chymotrypsin stain the tumor cell cytoplasm
♦
CK7, CA19-9, and mCEA are negative
Differential Diagnosis
♦
Ductal adenocarcinoma is positive for CK7, CA19–9, and mCEA and negative for α1–antitrypsin, lipase, trypsin, and chymotrypsin
♦
Neuroendocrine tumor is positive for pankeratin, chromogranin, synaptophysin, and CD56 and negative for α1–antitrypsin, lipase, trypsin, and chymotrypsin
♦
Solid pseudopapillary neoplasm is positive for α1-antitrypsin, PR (nuclear), β–catenin (nuclear), and CD56 and negative for pankeratin (weak, focal)
Neuroendocrine Neoplasms
♦
Pankeratin stains the tumor cell cytoplasm
♦
Synaptophysin, chromogranin (61%), and CD56 stain the cytoplasm focally or diffusely
♦
Chymotrypsin (30%) and α1-antitrypsin (38%) are focally positive
♦
Hormone markers can identify the cell type; this is most useful for identifying insulinoma due to its better prognosis
Differential Diagnosis
♦
Acinar cell carcinoma is positive for pankeratin, synaptophysin (67%), α1-antitrypsin, lipase, trypsin, and chymotrypsin; some cases are positive for CD56 (17%) and negative for chromogranin
♦
Invasive ductal carcinoma is positive for pankeratin and negative for synaptophysin, chromogranin, and CD56
♦
Lymphoma is positive for CD45 and CD3 or CD20; some types are positive for CD56 and negative for pankeratin, synaptophysin, and chromogranin
Solid Pseudopapillary Neoplasm
Verification Panel (Fig. 2.17)
♦
PR and β-catenin stain the tumor cell nuclei; PR also stains the benign islet cells
♦
CD10 stains the tumor cell membranes and cytoplasm
♦
Pankeratin shows focal weak or negative tumor cell cytoplasmic staining
♦
α1-antitrypsin, synaptophysin, vimentin, and CD56 stain the tumor cell cytoplasm
♦
Chromogranin is negative in the tumor cells
Fig. 2.17.
(A–D) Pankeratin stains the cytoplasm (weakly) and trypsin stains the intracytoplasmic globules in a solid pseudopapillary neoplasm. PR stains the nuclei of tumor cells (left) and benign islets (right).
Differential Diagnosis
♦
Acinar cell carcinoma is positive for pankeratin, synaptophysin (67%), α1-antitrypsin, lipase, trypsin, and chymotrypsin; some cases are positive for CD56 (17%) and negative for PR (nuclear) and β–catenin (nuclear)
♦
Invasive ductal carcinoma is diffusely positive for pankeratin and negative for PR (nuclear), β–catenin (nuclear), α1–antitrypsin, synaptophysin, and CD56
♦




Neuroendocrine tumor is positive for pankeratin, chromogranin, synaptophysin, α1-antitrypsin, and CD56 and negative for PR (nuclear) and β–catenin (nuclear)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


