, Tsunehisa Kaku2, Toru Sugiyama3 and Steven G. Silverberg4
(1)
Matsue City Hospital, Matsue, Shimane, Japan
(2)
Department of Health Sciences, Department of Health Sciences Graduate School of Medical Sciences, Fukuoka, Fukuoka, Japan
(3)
Department of Obstetrics and Gynecology, Iwate Medical University School of Medicine, Morioka, Iwate, Japan
(4)
Department of Pathology, University of Maryland School of Medicine, Baltimore, MD, USA
The immunohistochemical literature on ovarian clear cell carcinoma (CCC) is, like many other subjects, hampered by both small series of reported cases and the use of “house pet” antibodies which are generally unavailable in the outside world. Although our own series which forms the basis of this Atlas consists of more than 600 cases, the material available for centralized pathologic review consisted almost exclusively of hematoxylin- and eosin (H&E)-stained slides, so this chapter will consist predominantly of a brief review of the diagnostically and/or prognostically significant literature.
CCC shares with other ovarian epithelial carcinomas diffuse strong immunoreactivity for epithelial markers including CK7, CAM5.2, and EMA. These can occasionally be useful in the differential diagnosis with non-epithelial tumors featuring clear or oxyphil/oncocytic cells, but are not helpful in the distinction of CCC from primary ovarian serous, endometrioid, and mutinous carcinomas [1].
On the other hand, there is a subset of antibodies that has been found useful in these distinctions (Figs. 6.1 and 6.2) [1–4]. In a study of interobserver reproducibility in the diagnosis of ovarian carcinoma histotype, Kobel and colleagues [1] found a high level of interobserver agreement with the use of H&E slides alone but also noted significant improvement when an immunohistochemical panel consisting of WT-1, p53, p16, HNF-1 beta, ARID1A, and progesterone receptor (PR) was added to the review. Others have also confirmed these observations, particularly for CCC. In a study of 155 cases, DeLair and colleagues characterized the immunoprofile of CCC as HNF positive and ER/PR/WT-1/p53 negative [2]. As the only consistently positive marker for CCC in this group, HNF-1 beta (hepatocyte nuclear factor-1 beta) is particularly valuable. In one study [3], 21 of 22 CCC and only one of 61 non-CCC ovarian cancer specimens were found to have strong nuclear staining for this marker; the authors also confirmed upregulation of HNF-1 beta in CCC by real-time quantitative reverse transcription-polymerase chain reaction and immunoblotting and suggested HNF-1beta as a possible molecular target for CCC therapy.
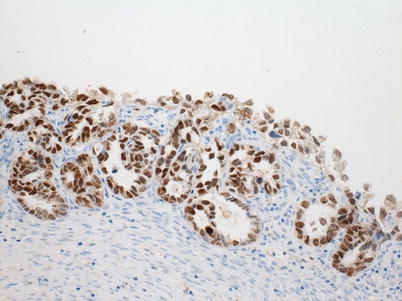
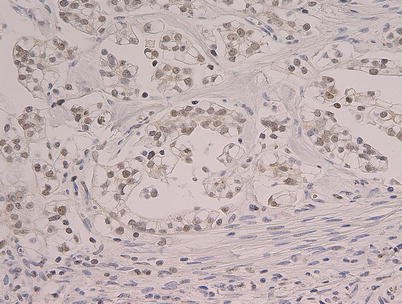

Fig. 6.1
Strongly positive HNF-1beta nuclear immunostaining in a clear cell carcinoma

Fig. 6.2




Diffuse moderate nuclear reactivity for ARID1A in a clear cell carcinoma. Note: sections of 4-mm thickness were cut from the paraffin blocks of CCC. Each section was mounted on a glass slide, deparaffinized, and soaked in 0.3 % H2O2/methanol for 15 min at room temperature to block endogenous peroxidase. HNF-1beta staining: the slides were pretreated with EnVisionTM FLEX Target Retrieval Solution, High pH (1:50) (DAKO) using PT-Link system (DAKO) for heat-induced epitope retrieval at 97 °C for 20 min. After blocking intrinsic peroxidases with 0.1 % H2O2, the slides were incubated with the goat antihuman HNF polyclonal antibody (C-20: Santa Cruz, sc-7411) (1:2,000) at 4 °C for 17 h. After washing out the extra primary antibodies by several rinses in PBS, the slides were incubated with the secondary antibody (rabbit anti-goat immunoglobulins (DAKO), 1:200) at room temperature for 30 min. The location of HNF-1beta on the slides was visualized with DAB after adding EnVisionTM FLEX + Rabbit (LINKER) (DAKO). ARID1A: a mouse monoclonal anti-ARID1A (1:500; Santa Cruz Biotechnology, Santa Cruz, CA) was applied for 2 h at 37 °C. The primary antibody was visualized using the Histofine Simple Stain PO (M) kit (Nichirei, Tokyo, Japan) according to the instruction manual. The slide was counterstained with hematoxylin
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


