Immunohistochemistry
Immunophenotypic analysis is extremely helpful and often essential for the diagnosis and classification of malignant neoplasms. Two major methods are used for determining the immunophenotype of neoplasms: immunohistochemistry and flow cytometry. The latter is discussed in Chapter 7. For the workup of hematologic neoplasms, either method can be applied. The advantages and disadvantages of each will be discussed. For the workup of solid tumors metastatic to lymph nodes, immunohistochemistry is used almost exclusively.
Metastatic Solid Tumors In Lymph Nodes
The identification of metastatic solid tumors in lymph nodes is one of the most important tasks in diagnostic surgical pathology. When regional lymph nodes are examined as part of a resection specimen, the primary neoplasm is known and the major task is to simply identify the presence of metastases. However, up to 5% of patients with solid tumors initially present with lymphadenopathy as a result of metastasis from an occult primary tumor (1,2)
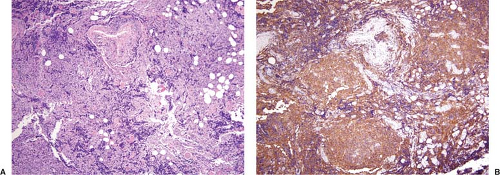
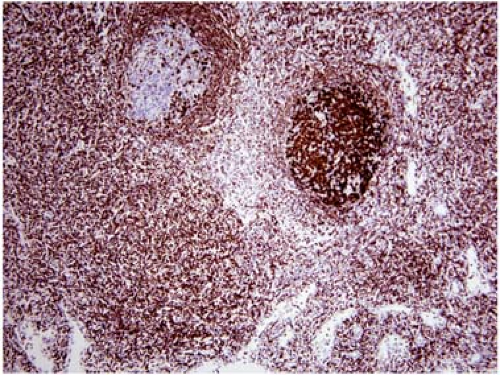
In many biopsy specimens, routine histologic examination provides clues to the site of origin (e.g., melanin pigment in malignant melanoma). However, poorly differentiated metastatic solid tumors lack these histologic clues. Although these neoplasms can be loosely grouped into categories based on their histologic features, such as epithelioid, anaplastic, spindle cell, or small-cell, the differential diagnosis of each of these categories is broad. Immunophenotypic analysis can add much information, thus increasing the pathologist’s ability to predict the site of origin. Either flow cytometry or immunohistochemistry can be used for this purpose. However, immunohistochemistry is ideally suited to the workup of metastatic solid neoplasms because these metastases often involve lymph nodes partially or focally, thereby making visual recognition of the metastasis and its immunoreactivity required (Fig. 6.1).
Many immunohistochemical stains can be used for identification and classification of metastatic neoplasms, and this number is growing (Table 6.1). In general, the antibodies used can be grouped into initial and secondary panels influenced, in large part, by the histologic features of the neoplasm (e.g. epithelioid, spindle cell, etc.). In general, the commonly used first-tier diagnostic antibodies are highly specific but variably sensitive for detection of particular tumor types. This group includes reagents specific for keratin (usually a cocktail of antikeratin antibodies), epithelial membrane antigen (EMA, also known as MUC1) S100 protein, vascular markers (CD31 and CD34), LCA (CD45RB), germ cell tumor markers (PLAP, OCT4), spindle cell tumor markers (e.g., desmin, smooth muscle actin), and plasma cell–associated markers (CD38, CD138).
In metastatic carcinomas of unknown origin, a second group of antibodies can be used to suggest a possible primary site. Currently, the keratin expression pattern is probably the most widely used, particularly those antibodies specific for keratins 7 and 20 (3). For example, gastrointestinal carcinomas are typically negative for keratin 7 and positive for keratin 20. Expression or lack thereof of other types of keratin is also helpful. Hepatocellular carcinoma is typically negative for keratin 19, in contrast with cholangiocarcinoma. The quality of keratin immunoreactivity also can be helpful. Punctate or dot-like cytoplasmic staining, as is commonly observed in Merkel cell carcinoma and small-cell carcinoma, is suggestive, but not specific for these neoplasms.
Other antibodies are also available that, when reactive, are more or less tumor-specific. Perhaps the best examples of these reagents are prostatic acid phosphatase and prostate-specific antigen. Additional specific antibodies are being developed or validated; RCC for renal cell carcinoma and HepPar for hepatocellular carcinoma are two examples. Other antibodies, although not specific, are suggestive of a limited number of possible primary sites. An example is estrogen receptor positivity which is suggestive of a breast or gynecologic-tract primary site.
Hematologic Neoplasms in Lymph Nodes
Immunophenotypic data are key components of the World Health Organization (WHO) lymphoma classification and necessary for the diagnosis of most specific lymphoma entities (4). Some lymphoma types have a characteristic immunophenotype, and recognition of this immunophenotype in the appropriate clinical and morphologic context strongly supports the diagnosis. Immunophenotype is also essential in the differential diagnosis of morphologically similar entities.
As stated earlier, two major methods are used for determining the immunophenotype of hematologic neoplasms: immunohistochemistry and flow cytometry (see Chapter 7). Flow cytometry immunophenotyping has many advantages, some of which include high sensitivity, simultaneous assessment of multiple antigens in a single cell, and the ability to analyze large numbers of cells rapidly and quantitatively. The major drawback of using flow cytometry is that one cannot directly visualize the architectural features of a biopsy specimen or the cytologic features of the cells being immunophenotyped. Although not an issue when a lymph node biopsy specimen is replaced by a homogeneous population of neoplastic cells, this drawback of flow cytometry can become an issue when the neoplasm represents only a small proportion of all cells in the biopsy specimen, especially when the cytologic features of the neoplastic cells must be correlated with the immunophenotype.
Circumstances in Which Immunohistochemistry is Often More Helpful than Flow Cytometry
There are a number of specific circumstances in which using immunohistochemistry for immunophenotyping is more advantageous than flow cytometry. As stated earlier, any neoplasm in which direct visual examination of the tumor architecture or the neoplastic cells themselves is helpful is a potential indication
for the use of immunohistochemistry. Other circumstances also arise when flow cytometry analysis is compromised for one reason or another, and immunohistochemistry can be used in its place. The following is a list of circumstances in which immunohistochemistry has advantages over flow cytometry. (Flow cytometry and its advantages are discussed in Chapter 7.) This list is meant to be illustrative, rather than a complete catalog of all possible circumstances.
for the use of immunohistochemistry. Other circumstances also arise when flow cytometry analysis is compromised for one reason or another, and immunohistochemistry can be used in its place. The following is a list of circumstances in which immunohistochemistry has advantages over flow cytometry. (Flow cytometry and its advantages are discussed in Chapter 7.) This list is meant to be illustrative, rather than a complete catalog of all possible circumstances.
TABLE 6.1 IMMUNOSTAINS USED TO IDENTIFY SITE OF ORIGIN OF METASTATIC Solid Tumors* | ||||||||||||||||||||||||||||||||||||||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Hodgkin lymphoma. This is perhaps the best example of a lymphoid neoplasm in which immunohistochemistry is more helpful than flow cytometry for diagnosis. In Hodgkin lymphoma, the neoplastic cells commonly represent 1% or less of all cells in a biopsy specimen. Identifying these large cells by flow cytometry is challenging, and is not successfully achieved using routine methods in clinical laboratories (5,6). In contrast, immunohistochemistry allows direct visual assessment of the lineage of the neoplastic cells.
Non-Hodgkin lymphomas in which the neoplastic cells represent a minority of the total cell population (e.g., T-cell/histiocyte-rich large B-cell lymphoma).
Partial or focal involvement by non-Hodgkin lymphoma. In this instance, unlike in the first two instances listed, the neoplasm involves only a small area of the biopsy specimen. As a result, the specimen submitted for flow cytometry may not contain the neoplasm. Alternatively, the neoplasm is included in the sample for flow cytometry but numerous reactive cells in the specimen obscure (or at least complicate) the analysis of the neoplastic cells.
Assessment of extranodal sites involved by lymphoma, particularly when the biopsy specimen is small. In some cases, the neoplastic cells are not well represented in cell suspensions prepared from extranodal sites, and the flow cytometry results are not representative.
Poor lymphoma cell viability. A nonviable or poorly viable specimen results in abundant debris and possibly an inadequate number of cells with appropriate surface antigen expression (6). A good example of this is infarcted lymphoma. Flow cytometry analysis of infarcted tissues usually does not yield interpretable results. Despite the poor tumor cell viability, intracellular antigens often are partially preserved, thus allowing immunohistochemical assessment of highly expressed antigens and tumor cell lineage.
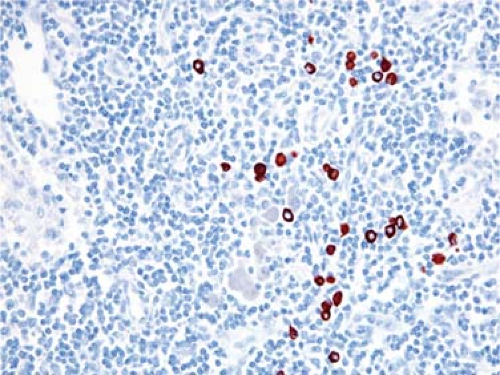
Assessment of cellular staining pattern. The location of antigen within a cell can provide useful information. For example, the pattern of anaplastic lymphoma kinase (ALK) immunoreactivity within anaplastic large-cell lymphoma correlates with the molecular abnormality; nuclear and cytoplasmic staining is highly predictive of t(2;5)(p23;q35), whereas cytoplasmic staining alone correlates with variant translocations involving ALK (reviewed in reference 7) (Fig. 6.2). Flow cytometry can detect ALK expression in ALCL but cannot determine the cellular location of the antigen.
Assessment of tumor architecture. Recognition of tumor architecture can be helpful for diagnosis and, in some tumors, assessment and quantification of tumor pattern is recommended. For example, in mantle cell lymphoma, the mantle zone pattern (Fig. 6.3) has prognostic meaning and can alter the treatment approach. In follicular lymphoma, the WHO classification recommends that the percentages of follicular and diffuse patterns be semiquantified (4). In addition, in follicular lymphoma, the extent of follicular pattern is often underappreciated in hematoxylin-eosin stained sections and is highlighted by immunostaining with antibodies specific for CD10, BCL-6, or pan–B-cell antigens (Fig. 6.4).
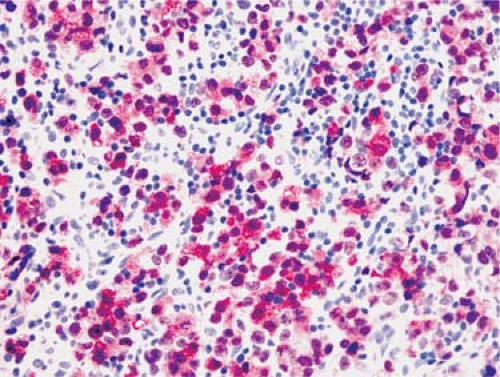
Assessment of nuclear antigens. A good example of a nuclear antigen best assessed by immunohistochemistry is cyclin D1 expression, usually in the context of the differential diagnosis of mantle cell lymphoma (Fig. 6.3). Currently, flow cytometry assays for assessing cyclin D1 are suboptimal because the antigen is intracellular and not expressed at very high levels. Terminal deoxynucleotidyl transferase (TdT) is
another nuclear antigen. As TdT is expressed more brightly than cyclin D1, it is easier to assess by flow cytometry with cell permeabilization techniques. However, cell permeabilization increases background staining (6), which makes the use of flow cytometry problematic when the neoplasm represents only a small percentage of all cells within a specimen.
Assessment of cytoplasmic antigens. Assessment of cytoplasmic immunoglobulins (Igs) in plasma cell myeloma is one of the more frequent applications requiring the assessment of intracytoplasmic antigens. Flow cytometry can easily identify plasma cells, as they are brightly positive for CD38 and CD138, and usually can successfully assess cytoplasmic Ig expression in plasma cell myeloma. In our experience, however, the assessment of cytoplasmic Ig by flow cytometry when the number of plasma cells is small (e.g., after therapy) can be misleading because of background staining as a result of cell permeabilization.
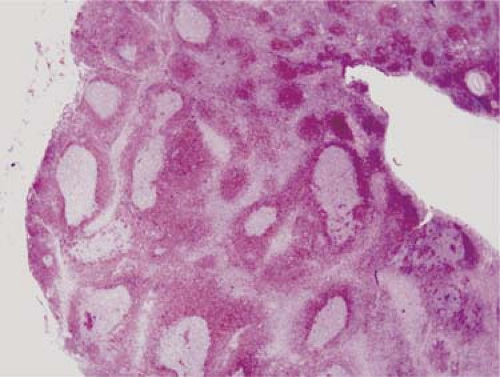
The differential diagnosis of follicular hyperplasia versus follicular lymphoma. Flow cytometry often can achieve this goal by showing polyclonal versus monoclonal immunoglobulin light chain expression. In some cases, for example in immunoglobulin-negative tumors, it is also helpful to examine the follicles for BCL-2 expression. BCL-2 can be assessed by flow cytometry, and this is routinely done in some clinical laboratories. In our experience, however, we also find that immunohistochemical analysis with direct visual assessment of the follicles for BCL-2 expression can be very helpful. This is particularly true in cases of so-called in situ follicular lymphoma (Fig. 6.5). BCL-2 is negative in the germinal centers of follicular hyperplasia and is usually positive in the follicles of follicular lymphoma (8).
The differential diagnosis of atypical Burkitt/Burkitt-like lymphoma and diffuse large B-cell lymphoma. The immunophenotype of these two neoplasms can be very similar. Immunohistochemical assessment of the extent and pattern of Ki-67 staining can be helpful. In Burkitt lymphoma, virtually all of the neoplastic cells are uniformly and strongly positive for Ki-67; this pattern is less common in diffuse large B-cell lymphoma (4).
Composite lymphoma. Knowledge of topography and the immunophenotype of both components is required and is better achieved by using immunohistochemistry.
Immunohistochemical Methods
Many techniques have been developed for immunohistochemical analysis and, in recent years, many steps in the process of immunostaining have become automated. Here, we briefly describe the principles of immunohistochemical analysis. For additional details, the reader is referred to a number of textbooks on immunohistochemistry.
The first step in immunohistochemical analysis is the application of a primary antibody. Polyclonal and monoclonal antibodies are used. Most polyclonal antibodies are derived from rabbits, whereas monoclonal antibodies are derived from mice. However, rabbit monoclonal antibodies have been recently developed that appear to be more sensitive than mouse monoclonal antibodies. If a mouse monoclonal antibody is used, the second step involves an anti-mouse immunoglobulin complexed with a large molecule that will allow amplification of the signal. In the commonly used avidin–biotin complex (ABC) immunoperoxidase method, the anti-mouse immunoglobulin is complexed with biotin. The third step involves adding avidin–biotin–horseradish peroxidase–antiperoxidase complex. The peroxidase then reacts with a chromogen to produce a reaction product that is discretely localized according to the normal site(s) of the cellular antigen being assessed. The reaction product may be localized to the surface, cytoplasm, or nucleus depending on the normal distribution of the antigen in the cell.
Using the ABC system, two chromogens are commonly used: diaminobenzidine (DAB) or 5–amino-9–ethyl carbazole (AEC). We prefer DAB because it yields a brown reaction product that fades little over time. AEC yields a red-brown reaction product that is aesthetically attractive, in the eyes of some more so than DAB, but the reaction product can fade over time as diffusion occurs, particularly if cover-slipped incorrectly.
The alkaline phosphatase-antialkaline phosphatase (APAAP) detection system also is commonly used for immunohistochemistry. Use of APAAP is advantageous because many types of cells have endogenous peroxidase (e.g., eosinophils) that complicate analysis using the ABC approach. Methods for blocking endogenous peroxidase can be used, but these methods can decrease the sensitivity of detecting certain antigens. Some cells have endogenous alkaline phosphatase, but generally in fewer cells than those that carry endogenous peroxidase, and the endogenous alkaline phosphatase is relatively easy to block. The APAAP and ABC methods can be combined for double immunolabeling.
Frozen Versus Routinely Processed Tissue Sections
Both frozen and fixed, paraffin-embedded tissue sections can be used for immunohistochemical analysis. To obtain the best results with frozen tissue, it is best to snap-freeze the tissue in isopentane/liquid nitrogen (or dry ice) or acetone/dry ice baths. Tissue frozen in OCT compound in a cryostat also can be used, although ice crystals are more likely to form and freeze-thaw artifact can destroy antigens. The great advantage of using frozen sections is that virtually every antibody used for flow cytometry immunophenotyping can be used for immunohistochemical analysis of frozen sections. Another advantage is that surface immunoglobulin expression by B-cells is not destroyed by the process of freezing tissue, and therefore clonality can be assessed. The disadvantages are mainly two: (a) frozen tissue is often not available, and (b) the architectural and cytologic features within the biopsy specimen are not as clearly seen as can be appreciated in routinely stained tissue sections (Fig. 6.6).
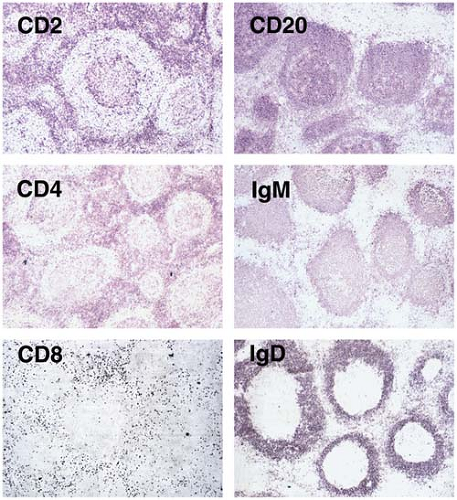 Figure 6.6. Reactive follicular hyperplasia assessed using immunohistochemistry and frozen tissue sections. Left column: CD2 is expressed by almost all T cells in the interfollicular zone and in the germinal center. CD4+ helper/inducer T cells, a major T-cell subset, localize to the interfollicular zone and germinal center. CD8+ suppressor/cytotoxic T cells, a lesser T-cell subset, are located mostly in the interfollicular zone. Right column: CD20 is expressed by almost all B cells, located predominantly in the mantle zone and follicle germinal centers. Both mantle zone and germinal-center B cells express membranous IgM, but only mantle zone B cells express IgD. Immunohistochemistry with aminoethyl carbazole as the chromogen and no counterstain.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|