Chapter 17 Immune Modifiers
Systemic Steroids
MOA (Mechanism of Action)
 Cortisol is the endogenous glucocorticoid and is synthesized from cholesterol. The hypothalamus secretes corticotropin-releasing hormone (CRH), which stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH), which acts on the adrenal glands to produce cortisol.
Cortisol is the endogenous glucocorticoid and is synthesized from cholesterol. The hypothalamus secretes corticotropin-releasing hormone (CRH), which stimulates the anterior pituitary to release adrenocorticotropic hormone (ACTH), which acts on the adrenal glands to produce cortisol. Cortisol acts in the nucleus of the cell; therefore it must first diffuse through the cell membrane. It is bound in the cytoplasm with heat shock protein and other proteins and transported into the nucleus.
Cortisol acts in the nucleus of the cell; therefore it must first diffuse through the cell membrane. It is bound in the cytoplasm with heat shock protein and other proteins and transported into the nucleus. Steroid receptors located in the nucleus are called glucocorticoid receptor elements. They regulate transcription of DNA by acting on promoters, which are specific DNA sites where RNA polymerase binds and starts transcription.
Steroid receptors located in the nucleus are called glucocorticoid receptor elements. They regulate transcription of DNA by acting on promoters, which are specific DNA sites where RNA polymerase binds and starts transcription. Steroids exert their actions in a broad range of tissues, and therefore there are many effects of glucocorticoids:
Steroids exert their actions in a broad range of tissues, and therefore there are many effects of glucocorticoids: Catabolism
CatabolismPharmacokinetics
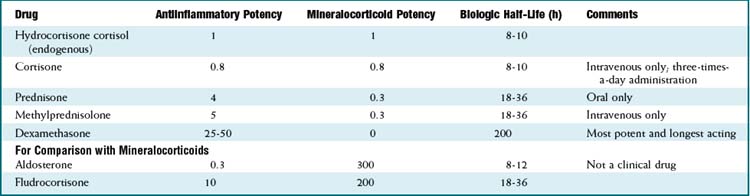
 Cortisol is largely bound to corticosteroid-binding globulin (CBG); it is also bound to albumin but with only low affinity (Table 17-1).
Cortisol is largely bound to corticosteroid-binding globulin (CBG); it is also bound to albumin but with only low affinity (Table 17-1).Side Effects
 Weight gain and severe swelling, particularly in the face; caused, in part, by the mineralocorticoid effects
Weight gain and severe swelling, particularly in the face; caused, in part, by the mineralocorticoid effects Psychiatric symptoms including depression, mania, and psychosis; other types of cognitive dysfunction can also occur, including euphoria, insomnia, mental confusion
Psychiatric symptoms including depression, mania, and psychosis; other types of cognitive dysfunction can also occur, including euphoria, insomnia, mental confusion Cushing’s syndrome is a collection of signs from endogenous overproduction of cortisol. A cushingoid appearance can be produced from iatrogenic exogenous steroid:
Cushing’s syndrome is a collection of signs from endogenous overproduction of cortisol. A cushingoid appearance can be produced from iatrogenic exogenous steroid: Adrenal suppression: Because of negative feedback loops, exogenous glucocorticoids will suppress CRH and ACTH. Rapidly terminating exogenous glucocorticoids after prolonged exposure will result in hypoadrenalism. Steroids must therefore be tapered (administration of smaller and smaller doses) before being stopped if they have been administered for more than about 1 or 2 weeks.
Adrenal suppression: Because of negative feedback loops, exogenous glucocorticoids will suppress CRH and ACTH. Rapidly terminating exogenous glucocorticoids after prolonged exposure will result in hypoadrenalism. Steroids must therefore be tapered (administration of smaller and smaller doses) before being stopped if they have been administered for more than about 1 or 2 weeks.Important Notes
 The baseline secretion of cortisol in the body is 10 to 20 mg. In periods of stress (illness, trauma, inflammation, infection), the secretion increases.
The baseline secretion of cortisol in the body is 10 to 20 mg. In periods of stress (illness, trauma, inflammation, infection), the secretion increases. Glucocorticoids must be differentiated from mineralocorticoids; the prototype mineralocorticoid is aldosterone, also a steroid, which acts primarily on the kidney to regulate water and electrolyte homeostasis.
Glucocorticoids must be differentiated from mineralocorticoids; the prototype mineralocorticoid is aldosterone, also a steroid, which acts primarily on the kidney to regulate water and electrolyte homeostasis. One significant side effect of glucocorticoids is an increased white blood count (driven by an increase in neutrophils). Increased neutrophil counts are more commonly caused by infection; because steroids increase the risk of infection, it is sometimes clinically difficult to differentiate whether the increased white count is the result of a new infection (which would make the steroids relatively contraindicated) or the result of a direct effect of the steroid. The rise in white cell count can be quite dramatic when caused by steroid effect alone.
One significant side effect of glucocorticoids is an increased white blood count (driven by an increase in neutrophils). Increased neutrophil counts are more commonly caused by infection; because steroids increase the risk of infection, it is sometimes clinically difficult to differentiate whether the increased white count is the result of a new infection (which would make the steroids relatively contraindicated) or the result of a direct effect of the steroid. The rise in white cell count can be quite dramatic when caused by steroid effect alone. Steroids and nonsteroidal antiinflammatory drugs (NSAIDs) are commonly coadministered for inflammatory conditions that result in pain (e.g., RA). The risk of gastrointestinal (GI) bleeding with steroids or NSAIDs alone is 2 times and 4 times (respectively) higher than baseline, but when these agents are taken together, the risk is 12 times higher than baseline.
Steroids and nonsteroidal antiinflammatory drugs (NSAIDs) are commonly coadministered for inflammatory conditions that result in pain (e.g., RA). The risk of gastrointestinal (GI) bleeding with steroids or NSAIDs alone is 2 times and 4 times (respectively) higher than baseline, but when these agents are taken together, the risk is 12 times higher than baseline. Because of the long list of side effects, steroids are not ideal for long-term high-dose administration. In diseases in which prolonged and substantial immunosuppression is required, steroid-sparing immunosuppressants are administered so that doses of steroids can be reduced or the steroids can be completely discontinued.
Because of the long list of side effects, steroids are not ideal for long-term high-dose administration. In diseases in which prolonged and substantial immunosuppression is required, steroid-sparing immunosuppressants are administered so that doses of steroids can be reduced or the steroids can be completely discontinued.Advanced
 Prophylactic antibiotics against Pneumocystis pneumonia (also called Pneumocystis jiroveci pneumonia or Pneumocystis carinii pneumonia [PCP]) are administrated to patients on long-term moderate- to high-dose steroids because of the risk of acquiring this infection when immunosuppressed. Human immunodeficiency virus (HIV) infection is the most common risk factor for PCP
Prophylactic antibiotics against Pneumocystis pneumonia (also called Pneumocystis jiroveci pneumonia or Pneumocystis carinii pneumonia [PCP]) are administrated to patients on long-term moderate- to high-dose steroids because of the risk of acquiring this infection when immunosuppressed. Human immunodeficiency virus (HIV) infection is the most common risk factor for PCP In addition to being a risk factor for PCP, steroids are also coadministered to patients being treated for PCP because the antibiotic-mediated destruction of the pathogen generates a strong inflammatory response in the lungs, making the respiratory condition much worse. Steroids blunt this response.
In addition to being a risk factor for PCP, steroids are also coadministered to patients being treated for PCP because the antibiotic-mediated destruction of the pathogen generates a strong inflammatory response in the lungs, making the respiratory condition much worse. Steroids blunt this response.Evidence
 Systemic steroids and adult asthma: A systematic review in 2009 concluded that the available studies were frequently underpowered. However, some general conclusions and recommendations were made: steroids administered in the emergency department reduce hospitalizations; steroids accelerate improvements in lung function; there was no benefit in using doses larger than 50 to 100 mg prednisone equivalent; and no benefit was seen when steroids were administered for longer than 5 to 10 days total.
Systemic steroids and adult asthma: A systematic review in 2009 concluded that the available studies were frequently underpowered. However, some general conclusions and recommendations were made: steroids administered in the emergency department reduce hospitalizations; steroids accelerate improvements in lung function; there was no benefit in using doses larger than 50 to 100 mg prednisone equivalent; and no benefit was seen when steroids were administered for longer than 5 to 10 days total. Systemic steroids and RA: A Cochrane review in 2007 (15 studies, N = 1414 patients) examined radiological progression of disease and concluded that the proportion of benefit gained by glucocorticoids in reducing the progression of erosions from an average of all the studies over 1 year was 67.2% (confidence interval [CI] 48.9%, 85.4%) and over 2 years was 61.3% (CI 46.5%, 76.1%). Furthermore, this benefit was achieved in patients who were (mostly) already receiving disease-modifying antirheumatic drug (DMARD) treatment. It therefore represents a gain over and above any benefits from DMARDs alone.
Systemic steroids and RA: A Cochrane review in 2007 (15 studies, N = 1414 patients) examined radiological progression of disease and concluded that the proportion of benefit gained by glucocorticoids in reducing the progression of erosions from an average of all the studies over 1 year was 67.2% (confidence interval [CI] 48.9%, 85.4%) and over 2 years was 61.3% (CI 46.5%, 76.1%). Furthermore, this benefit was achieved in patients who were (mostly) already receiving disease-modifying antirheumatic drug (DMARD) treatment. It therefore represents a gain over and above any benefits from DMARDs alone.Introduction to Monoclonal Antibodies
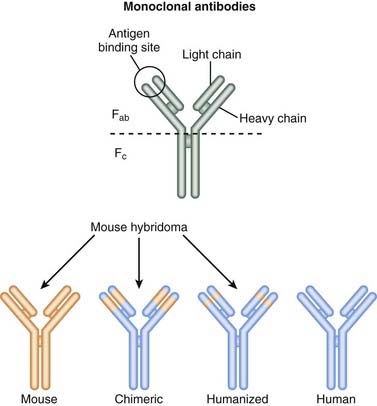
Newer technologies are enabling the animal (usually mouse) portion of the antibody to be less and less, so that the resulting antibody is mostly human and therefore not destroyed by the patient’s own immune system for being a foreign antibody by human antimurine antibodies (HAMAs). Chimeric (human-mouse combination) antibodies contain fewer mouse regions than full mouse antibodies. Humanization involves replacing most of the mouse antibody with equivalent human regions while keeping only the variable, antigen-specific regions intact. Humanized mAbs have more human regions than chimeric mAbs do. Finally, fully human mAbs that contain no mouse regions are now being created (Figure 17-1).
Fusion Proteins
Use of Monoclonal Antibodies
The list of uses for mAbs is growing, but the major categories are as follows:
 Cancer: Cancer cells express unique antigens that can be directly targeted for destruction. Furthermore, there are growth factors that stimulate cancer cell growth that can also be inhibited.
Cancer: Cancer cells express unique antigens that can be directly targeted for destruction. Furthermore, there are growth factors that stimulate cancer cell growth that can also be inhibited. Immunosuppressants:
Immunosuppressants: Inflammatory diseases: Autoimmune diseases in which the immune system attacks self tissues and cells, such as lupus and RA, have been treated with mAbs that target specific components of the immune system such as TNF and many of the interleukins (ILs) in an attempt to blunt the immune response.
Inflammatory diseases: Autoimmune diseases in which the immune system attacks self tissues and cells, such as lupus and RA, have been treated with mAbs that target specific components of the immune system such as TNF and many of the interleukins (ILs) in an attempt to blunt the immune response. Infection treatment: By binding specific functional proteins expressed on bacteria or viruses, the infectious agent can be suppressed or controlled.
Infection treatment: By binding specific functional proteins expressed on bacteria or viruses, the infectious agent can be suppressed or controlled.Naming Monoclonal Antibodies
Targets
 Disease processes or tissues: immune = lim, ILs = kin, viral = vir, bacterial = bac, infectious lesions = les, cardiovascular = cir, fungal = fung, neurologic = ner, musculoskeletal = mul, bone = os, toxin as target = toxa
Disease processes or tissues: immune = lim, ILs = kin, viral = vir, bacterial = bac, infectious lesions = les, cardiovascular = cir, fungal = fung, neurologic = ner, musculoskeletal = mul, bone = os, toxin as target = toxaNotes
Table 17-2 will not be fully inclusive by the time it is published because of the rapid growth of this area of medicine. Some drugs listed may not yet be approved for use.
Table 17-2 Monoclonal Antibodies
| Name | Type | Target |
|---|---|---|
| Rituximab | Chimeric | CD20 on B lymphocytes |
| Ocrelizumab | Humanized | CD20 on B lymphocytes |
| Ofatumumab | Human | CD20 on B lymphocytes |
| Tositumomab | Mouse | |
| Ibritumomab | Mouse | |
| Epratuzumab | Humanized | CD22 on B lymphocytes |
| Alemtuzumab | Humanized | CD52 on B and T lymphocytes |
| Muromonab | Mouse | CD3 on T lymphocytes |
| Efalizumab | Humanized | CD11a on T lymphocytes |
| Inolimomab | Mouse | CD25 (IL-2) on T lymphocytes |
| Basiliximab | Chimeric | CD25 (IL-2) on T lymphocytes |
| Daclizumab | Humanized | CD25 (IL-2) on T lymphocytes |
| Gemtuzumab | Humanized | CD33 on hematopoietic cells |
| Bevacizumab | Humanized | Vascular-endothelial growth factor (VEGF) |
| Ranibizumab | Humanized | Vascular-endothelial growth factor (VEGF) |
| Cetuximab | Chimeric | Epidermal growth factor receptor (EGFR) |
| Satumomab | Mouse | Tumor-associated glycoprotein TAG-72 |
| Capromab | Mouse | PSA (prostate), bound to 111In |
| Omalizumab | Humanized | IgE (increased in asthma) |
| Eculizumab | Humanized | C5 (complement) |
| Palivizumab | Humanized | Fusion protein of RSV |
| Trastuzumab | Humanized | HER2 (EGF) |
| Infliximab | Chimeric | TNF-α |
| Adalimumab | Human | TNF-α |
| Certolizumab pegol | Humanized | TNF-α, bound to polyethylene glycol |
| Denosumab | Human | Receptor activator for nuclear factor κ B ligand (RANKL) in bone |
EGF, epidermal growth factor; HER2, human epidermal growth factor receptor 2; 131I, iodine-131; IgE, immunoglobulin E; IL, interleukin; 111In, indium-111; PSA, prostate-specific antigen; TNF, tumor necrosis factor; 90Y, yttrium-90.
B-Cell Biologics
Description
B-cell biologics specifically target B-cell lymphocytes for either destruction or suppression.
MOA (Mechanism of Action)
 Drugs that target B cells cause either destruction of the cells or interference with their ability to mount an immune response.
Drugs that target B cells cause either destruction of the cells or interference with their ability to mount an immune response. As a B cell–depleting agent (destruction of B cells):
As a B cell–depleting agent (destruction of B cells): CD20 is implicated in antibody-dependent cytotoxicity, complement-dependent cytotoxicity, and induction of apoptosis of B lymphocytes.
CD20 is implicated in antibody-dependent cytotoxicity, complement-dependent cytotoxicity, and induction of apoptosis of B lymphocytes. Accelerated destruction by other anticancer drugs has also been observed when used in combination with CD20 therapy.
Accelerated destruction by other anticancer drugs has also been observed when used in combination with CD20 therapy. As an immunomodulator (inhibitor of B cells):
As an immunomodulator (inhibitor of B cells): B-cell depletion (through the cytotoxic actions described previously) is an effective method of blunting a pathologic inflammatory response.
B-cell depletion (through the cytotoxic actions described previously) is an effective method of blunting a pathologic inflammatory response. B cells play a pivotal role in the development and progression of many autoimmune diseases. B cells must go through a series of steps before they become immunologically active, and many of these steps require cytokine binding and stimulation. Therefore blocking these cytokines can result in inhibition of the following B cell functions:
B cells play a pivotal role in the development and progression of many autoimmune diseases. B cells must go through a series of steps before they become immunologically active, and many of these steps require cytokine binding and stimulation. Therefore blocking these cytokines can result in inhibition of the following B cell functions: Bound to radioactive ligands: When bound to radioactive ligands, the mAb delivers targeted radiotherapy to the target cells and destroys the cells through close contact with the radioactive ligand.
Bound to radioactive ligands: When bound to radioactive ligands, the mAb delivers targeted radiotherapy to the target cells and destroys the cells through close contact with the radioactive ligand. CD20 is expressed only on B cells and is present on all stages of B-cell differentiation except the very first and very last stages.
CD20 is expressed only on B cells and is present on all stages of B-cell differentiation except the very first and very last stages.Pharmacokinetics
 The half-life of rituximab can be variable when treating tumor. The drug will become bound and removed from circulation when a high number of CD20 receptors are present, effectively decreasing the drug concentration and half-life. Therefore at the start of cancer therapy the half-life is around 75 hours, whereas at the end of therapy it can be as long as 200 hours.
The half-life of rituximab can be variable when treating tumor. The drug will become bound and removed from circulation when a high number of CD20 receptors are present, effectively decreasing the drug concentration and half-life. Therefore at the start of cancer therapy the half-life is around 75 hours, whereas at the end of therapy it can be as long as 200 hours.Indications
 Inflammatory autoimmune diseases (inhibiting or depleting B cells will potentially reduce inflammation and the disease process):
Inflammatory autoimmune diseases (inhibiting or depleting B cells will potentially reduce inflammation and the disease process):Contraindications
 Active infection: These drugs are immunosuppressants and therefore would inhibit the body’s ability to fight off an infection.
Active infection: These drugs are immunosuppressants and therefore would inhibit the body’s ability to fight off an infection.Side Effects
Early
Important Notes
 CD20 antigen is not expressed by either plasma cells or B-lymphoid stem cells; therefore rituximab does not reduce total immunoglobulin (Ig) serum concentrations.
CD20 antigen is not expressed by either plasma cells or B-lymphoid stem cells; therefore rituximab does not reduce total immunoglobulin (Ig) serum concentrations.Advanced
 B cell–depleting and B cell–nondepleting strategies have both been used for treatment of autoimmune diseases.
B cell–depleting and B cell–nondepleting strategies have both been used for treatment of autoimmune diseases. Future therapies:
Future therapies: BAFF (B-cell activating factor), also called BlyS (B-lymphocyte stimulator), prolongs the survival of B cells, stimulates maturation, and promotes survival of autoreactive B. Anti-BAFF antibodies (belimumab) or BAFF receptor fusion proteins (briobacept) are being investigated for treating RA and SLE.
BAFF (B-cell activating factor), also called BlyS (B-lymphocyte stimulator), prolongs the survival of B cells, stimulates maturation, and promotes survival of autoreactive B. Anti-BAFF antibodies (belimumab) or BAFF receptor fusion proteins (briobacept) are being investigated for treating RA and SLE.T-Cell Biologics
Description
T-cell biologics specifically target T-cell lymphocytes for either destruction or suppression.
MOA (Mechanism of Action)
 The roles and activities of T cells are extremely diverse and complicated. Their contributions to disease are currently incompletely understood, and this section is a very simplified explanation of a very complicated system. This area of medicine is changing quickly, as is the understanding of these disease processes.
The roles and activities of T cells are extremely diverse and complicated. Their contributions to disease are currently incompletely understood, and this section is a very simplified explanation of a very complicated system. This area of medicine is changing quickly, as is the understanding of these disease processes.