Hybrid and potentially pathogenic Escherichia coli strains
Victor A. Garcia-Angulo1, Mauricio J. Farfan2 and Alfredo G. Torres1, 1University of Texas Medical Branch, Galveston, TX, USA, 2Universidad de Chile, Santiago, Chile
Diffusely adherent E. coli (DAEC)
Background
Definition
Diffusely adherent E. coli (DAEC) is one of six classical pathotypes of diarrheagenic E. coli (DEC), with the ability to adhere over the entire surface of HEp-2/HeLa cells in a diffuse adherent (DA) pattern (Scaletsky et al., 1984). Analysis of the virulence determinants of DAEC strains indicated that they are a diverse group of strains with virulence genes homologous to those found in DEC strains (EAEC, ETEC, or EPEC) or in extraintestinal E. coli strains associated with urinary tract infections (UTIs) (Czeczulin et al., 1999; Servin, 2005).
History and evolution
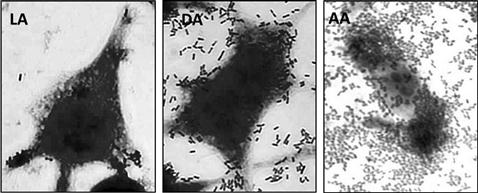
To recognize DAEC strains from diarrheal stool samples of patients, the ability of the bacteria to adhere to HEp-2/HeLa cells has been used (Mathewson and Cravioto, 1989). This assay differentiated three adherence phenotypes: localized, diffuse, or aggregative adherence (LA, DA, AA, respectively; Figure 11.1). Analysis of the factors involved in the DA pattern led to the discovery of two adhesins, the F1845 fimbriae and AIDA-I autotransporter (Benz and Schmidt, 1989; Bilge et al., 1989). Since then, DNA probes and PCR specific for these virulence factors have been used for the identification of DAEC strains (Jallat et al., 1993; Vidal et al., 2005), and to design epidemiological studies associating DAEC with diarrhea cases. In recent years, several groups have focused on the study of F1845 fimbriae producing strains, since these fimbriae are related to the Afa/Dr adhesins, which are expressed in a variety of pathogenic genetic backgrounds (Escobar-Paramo et al., 2004), and have been associated with enteric and urinary tract infections.
Epidemiology and global impact
There are several studies that investigated the involvement of DAEC in diarrheal disease and, overall, they indicated that this association is still unsettled. Prevalence of DAEC isolates in stool samples of children with diarrhea is low compared to other DEC pathotypes (Gomez-Duarte et al., 2010; Rajendran et al., 2010). Some studies have associated DAEC strains with diarrheal disease in infants, children, and adults (Giron et al., 1991; Jallat et al., 1993). Further, an age-related incidence of DAEC strains expressing Afa/Dr adhesins has been described, implicating DAEC as a cause of diarrhea, particularly in children >12 months of age (Gunzburg et al., 1993; Levine et al., 1993). On the other hand, no differences in the frequency of DAEC strains were found in case-control studies and no relation with the type or duration of diarrhea in children was attributable to DAEC (Gomes et al., 1989; Cravioto et al., 1991). More strikingly, volunteer studies showed that DAEC strains orally administered failed to induce diarrhea in adults (Tacket et al., 1990).
A completely different situation is observed for DAEC strains associated with UTI infections. Afa/Dr DAEC strains are involved in 25–50% of cystitis cases in children, 30% of pyelonephritis cases in pregnant women, and one-third of recurrent UTIs in adults (Archambaud et al., 1988; Daigle et al., 1994). Moreover, a characterization of urinary E. coli isolates from 174 women with a first episode of UTI and assessment of the risk of a second UTI indicated that Afa/Dr adhesins were associated with a two-fold increased risk (Foxman et al., 1995). These observations have led to investigations about the function of Afa/Dr adhesins in DAEC pathogenicity, to understand the mechanism of DAEC interactions with the epithelia (Servin, 2005Le Bouguenec and Servin, 2006).
Molecular pathogenesis
Mechanism of pathogenicity
Adherence to epithelial cells is probably the most important step in DAEC infection. This process includes highly specific interaction with the epithelium to: (i) recognize the infection site; (ii) compete and gain access to gastrointestinal and urinary tract regions that are occupied by the resident microflora; and (iii) activate signal transduction cascades that are necessary to induce diarrhea and/or inflammation (Benz and Schmidt, 1989; Bilge et al., 1989; Betis et al., 2003a,b). Many factors can contribute to DAEC adhesion to the host cells and some adhesins have been characterized (Betis et al., 2003a,b). Two main adhesins have been identified in DA strains, the adhesin involved in diffuse adherence (AIDA-1) and the F1845 fimbriae (Benz and Schmidt, 1989; Bilge et al., 1989). AIDA-1 is a plasmid-encoded 100 kDa autotransporter protein which was initially identified in an EPEC strain mediating adherence to HeLa cells and displaying the DA phenotype (Benz and Schmidt, 1989; Maurer et al., 1997). The nucleotide sequence of the F1845 fimbriae has significant homology with the Afa/Dr family of adhesins, which are virulence determinants in uropathogenic E. coli strains (Nowicki et al., 2001). Considering that approximately 75% of DAEC strains produce the F1845 or a related adhesin (Bilge et al., 1989), the role of Afa/Dr adhesins on the DAEC pathogenesis has been the focus of several studies.
The Afa/Dr adhesin family contains representatives of fimbrial and non-fimbrial structures, organized in operons consisting of at least five genes and belonging to the chaperone-usher family of adhesins. These operons are present in both diarrheal and uropathogenic E. coli strains and encode for the Afa/Dr adhesin, an invasin, and proteins required for the secretion and assembly of the adhesin at the bacterial cell surface (see Chapter 12) (Servin, 2005; Le Bouguenec and Servin, 2006). Afa/Dr adhesins are also classified on the basis of the host cell receptors that they bind, such as the Dr(a) blood-group antigen present on the decay-accelerating factor (DAF, CD55), the extracellular matrix protein collagen IV and/or carcinoembryonic antigen-related cellular adhesion molecules (CEACAMs) (Nowicki et al., 2001; Berger et al., 2004). All these receptors play a pivotal role in DEAC adherence to the gastrointestinal and urinary tract.
Adherence
Using polarized epithelial cultured cells it was established that Afa/Dr adhesins interact with DAF or CEACAM receptors (CEACAM6 for F1845) and, as a result, the receptors accumulate underneath the bacteria (Goluszko et al., 1999; Le Bouguenec et al., 2001). This interaction also triggers signal transduction cascades that control the growth of long finger-like cellular projections (lamellipodia) which wrap around the bacteria, promoting tight attachment of the bacteria to the cell surface (Cookson and Nataro, 1996). In addition, DAEC adhesion is accompanied by brush border lesion, F-actin rearrangement and tight junction disruption (Bernet-Camard et al., 1996; Peiffer et al., 2000). As a result of the adherence process, the bacteria become firmly attached to the cell surface, initiating the colonization steps and activation of signal transduction pathways, resulting in the internalization of the bacteria and/or inflammatory responses.
Internalization
A small proportion of adherent Afa/Dr DAEC strains are able to invade cultured epithelial cells, a process that is also associated with the Afa/Dr adhesins (Guignot et al., 2009). During in vitro culture, Afa/Dr DAEC invades epithelial cells by a zipper-like mechanism, with the participation of microtubules, lipid rafts and α5β1 integrins (Goluszko et al., 1999; Kansau et al., 2004; Guignot et al., 2009). Once in the cytoplasm, internalized bacteria reside in a large vacuole formed by the fusion of single bacterium-containing vacuoles originated early during the initial step of invasion (Plancon et al., 2003; Servin, 2005).
Inflammation
DAEC infection is characterized by the induction of an inflammatory response on epithelial cells. Afa/Dr DAEC infection of intestinal cultured cells induces the release of pro-inflammatory factors, such as IL-8, as a result of the activation of the mitogen-activated protein kinases pathway (Betis et al., 2003a; Arikawa et al., 2005). The inflammation induced by Afa/Dr DAEC promotes polymorphonuclear migration across the epithelial barrier, inducing the production of TNF-α and IL-1β, which stimulate the production of DAEC receptor DAF (Betis et al., 2003b). In addition, Afa/Dr DAEC increase the cell-surface expression of the major histocompatibility complex class I chain-like gene A, a key factor in the host innate immune response (Tieng et al., 2002). Overall, these data provide a mechanism by which Afa/Dr DAEC may participate in the development of inflammatory diarrhea in humans.
Clinical manifestations
Transmission and clinical features
Acquisition through food or water contaminated with human or animal feces comprises the main transmission route for this pathovar. Clinical presentation of diarrhea episodes caused by diarrhea-causing DAEC might include watery or bloody diarrhea, abdominal cramps, dehydration and fever (Gunzburg et al., 1993; Jallat et al., 1993); however, a unique clinical characteristic specific for DAEC intestinal infection has not been described. Interestingly, the finding that Afa/Dr DAEC adhesins are carried by E. coli causing UTIs supports the hypothesis of fecal–perineal–urethral transmission as the etiology for these infections (Daigle et al., 1994; Foxman et al., 1995).
Diagnosis
At first, the HEp-2/HeLa cells adherence assay was widely used to identify DAEC strains. However, the identification and characterization of F1845 and AIDA-1 led to the use of these adhesins as markers for DAEC strains. It is important to note that a significant number of strains with a DA phenotype are negative for both markers and therefore are not classified as DAEC (Jallat et al., 1993, 1994). Therefore, additional studies to identify novel, more reliable markers for the DAEC pathotype are warranted. Considering that the prevalence of Afa/Dr adhesins in DAEC is higher than that of AIDA-I, numerous assays to diagnose Afa/Dr DAEC have been developed using the F1845 or related adhesins. Among these assays, the molecular detection of genes located in the Afa/Dr operon has been explored. Different DNA probes for colony hybridization have been assayed, but this is a time-consuming technique that requires trained personnel, making difficult its implementation in a clinical laboratory. PCR-based techniques, such as multiplex PCR, to identify E. coli colonies obtained from cultures or stool samples offer a more practical, rapid and accurate diagnosis. Multiplex PCR assay to identify all six E. coli pathotypes has provided a valuable tool for epidemiological studies (Vidal et al., 2005).
Treatment
Diarrheal illness caused by DAEC, as well as other DEC pathotypes, might be ameliorated by specific antimicrobials, however clinical data demonstrating their efficacy are lacking. Moreover, DAEC strains are associated with a high frequency of antibiotic resistance. DAEC strains have presented high levels of resistance to antibiotics commonly used to treat enteric infections, compared to the rest of DEC strains (Ochoa et al., 2009). Moreover, the majority of the DAEC strains with resistance to multiple antibiotics are associated with conjugative plasmids, a situation that not only hinders the antibiotic treatment, but also facilitates the dissemination of the antibiotic resistance genes between enteric pathogens (Lopes et al., 2005). In many countries, the implementation of oral rehydration is the treatment of choice for DEC infections, including DAEC. This strategy has significantly reduced deaths caused by diarrhea, but in severe cases, an antibiotic treatment might be necessary. In this case, the knowledge of the profile of antibiotic susceptibility can guide the selection of an adequate antimicrobial management. UTIs caused by DAEC are treated according to guidelines for other E. coli UTIs (see Chapter 9).
Immune response
In order to investigate the role of DAEC in the development of diarrhea, volunteers were fed reference DAEC strains. None of them developed diarrhea and only of small number developed IgG or IgA antibodies to DAEC (Tacket et al., 1990). These results and the controversial role of DAEC as a diarrhea-causing pathogen have reduced the interest to analyze the immune response against this pathotype and the development of strategies to control DAEC infections. On the other hand, the data indicating that Afa/Dr DAEC strains induce an inflammatory response on polarized intestinal cells (Betis et al., 2003a), and the increasing number of studies associating the Afa/Dr adhesin with UTI, should lead to a renewed interest to understand the immune response of DAEC in humans.
Control and prevention
General guidelines for management of patients with diarrhea are also recommended for DAEC infections. Patients admitted to hospital with diarrhea should be barrier-nursed, preferably in a side-room, and the infection control department should be notified. To prevent UTIs, the most important recommendation is to practice good personal hygiene. Antibiotic prophylaxis remains unclear and contradictory (Greenfield, 2011).
Adherent and invasive E. coli (AIEC)
Background
Definition and/or classification
Inflammatory bowel disease (IBD), comprises both Crohn’s disease (CD) and ulcerative colitis (UC), and affects approximately 2.6 million people in the US and Europe (Loftus, 2004). CD is a chronic, granulomatous inflammatory condition characterized by a strong activation of the immune system. Although CD can affect any site of the intestinal tract, it is more common in the ileum. CD causes erosions in the inner surface of the intestine known as aphthous ulcers (Kaser et al., 2010), which in severe cases can lead to intestinal obstruction, among other complications. A complex interplay of genetic and environmental factors contributes to triggering abnormal immune responses in CD (Hugot et al., 2001; Ogura et al., 2001; Helbig et al., 2012). However, cumulative evidence suggests that intestinal bacteria play a major role in the onset and perpetuation of the disease (Sartor, 2005; Rolhion and Darfeuille-Michaud, 2007; Carvalho et al., 2009; Flanagan et al., 2011). A significant reduction in the gut microbiota diversity with an over-representation of Enterobacteriaceae, mainly E. coli, is observed in patients with CD (Kotlowski et al., 2007; Flanagan et al., 2011; Joossens et al., 2011). Although belonging to different serogroups, E. coli isolates from CD patients have highly related ribotype profiles and most of them belong to the B2 and D phylogroups (Kotlowski et al., 2007; Joossens et al., 2011). Unlike non-pathogenic commensal E. coli, isolates from CD lesions have been shown to efficiently adhere to, invade and survive inside epithelial cells and macrophages. As such, this group of isolates has been collectively named adherent-invasive E. coli (AIEC) (Darfeuille-Michaud et al., 2004). AIEC have been isolated from up to one-third of the ileal lesions of CD patients. Thus, AIEC comprise an E. coli category associated with persistence in these multifactorial CD. The criteria for inclusion in the AIEC category include: (i) ability to adhere to and invade intestinal epithelial cells with a macropinocytosis-like entry process; (ii) ability to survive and replicate within macrophages without triggering host cell death; and (iii) ability to induce the release of large amounts of TNF-α from infected macrophages (Darfeuille-Michaud, 2002; Darfeuille-Michaud et al., 2004).
History
In 1932, Dr. Burrill B. Crohn and colleagues presented a paper to the American Gastro-Enterological Association entitled ‘Non-specific Granulomata of the Intestine’, describing the features of what is now known as CD, a disease different from UC (Baron, 2000; Naser et al., 2012). It was speculated that an infectious agent could account for an environmental cause of the disease. In 1970, Rees and Mitchel showed that mice inoculated with CD tissue displayed focal granulomas (Mitchell and Rees, 1970). Subsequently, Cave and collaborators demonstrated that rabbits inoculated with human CD tissue homogenates developed granuloma (Cave et al., 1973) and this effect could be prevented by ampicillin pretreatment of the homogenates (Donnelly et al., 1977), suggesting the presence of a transmissible agent in the disease. Other studies showed that antibody titers against E. coli antigens were higher in CD patients than in controls (Brown and Lee, 1973; Tabaqchali et al., 1978) and that most CD tissue samples were positively labeled by anti-E. coli antibodies (Cartun et al., 1993; Liu et al., 1995). E. coli were recovered frequently from CD tissues (Darfeuille-Michaud et al., 1998), and these isolates were shown to be genetically related by their ribotype profiles (Masseret et al., 2001), and able to adhere to differentiated intestinal epithelial cells more frequently than isolates from healthy tissues (Darfeuille-Michaud et al., 1998; Masseret et al., 2001). Additionally, the reference AIEC LF82 strain was shown to invade and replicate inside intestinal epithelial cells, using a process dependent on a macropinocytosis-like mechanism (Darfeuille-Michaud, 2002). Based on those characteristics, the designation of adherent-invasive E. coli as a new group of E. coli associated with CD was proposed (Darfeuille-Michaud, 2002).
Evolution
The onset and perpetuation of CD has not been associated with any particular E. coli strain, but the use of culture-independent methods of identification suggested that most E. coli strains colonizing CD lesions are genetically related. Ribotyping of E. coli isolates from CD patients with early, recurrent, or chronic lesions, with or without endoscopic recurrence showed that, while compared to healthy controls, most E. coli from CD cases grouped in a single ribotype profile (Masseret et al., 2001). E. coli species comprises four phylogenetic groups: A, B1, B2, and D, and the CD-specific isolates were found to belong to B2 and D groups (Kotlowski et al., 2007). A recent study did not find any phylogenetic relationship among CD E. coli isolates and most of the phylogenetic groups were found equally distributed in CD patients and healthy individuals; however, the B2 phylogroup was more prevalent among AIEC-positive isolates (Martinez-Medina et al., 2009).
Phylogenetic analyses based on multilocus sequence typing (MLST) showed that AIEC isolates cluster with the uropathogenic E. coli (UPEC) strain CFT073 and the avian pathogenic E. coli (APEC) serotype O1:K1:H7 (Sepehri et al., 2009). The relationship between AIEC and either UPEC or APEC strains could be further validated because AIEC strains encode and express UPEC-related adhesins and autotransporter proteins (Darfeuille-Michaud et al., 1998; Kotlowski et al., 2007). Phylogenetic analyses and full genome sequence of prototype AIEC strains confirmed relatedness to these strains and expanded the spectrum of putative virulence factors, either AIEC-specific or shared with UPEC and APEC (Miquel et al., 2010a; Nash et al., 2010; Clarke et al., 2011; Krause et al., 2011). The genomic sequence of AIEC LF82 reveals that this strain shares a high percentage of common coding sequences with APEC-01 strain and that the putative virulence determinants include gene products promoting motility, serum resistance, iron uptake, capsule and LPS expression, as well as biofilm formation, adhesion to and invasion of epithelial cells (Miquel et al., 2010b). AIEC NRG857c strain also clusters in the B2 phylogroup with APEC-01 and the UPEC strains 536 and CFT073, and genomic analysis showed considerable sequence similarity and synteny with AIEC LF82 (Nash et al., 2010). Overall, AIEC strains seem to be evolutionarily and genetically related to extraintestinal E. coli strains, suggesting that both pathovars might use similar pathogenic strategies.
Epidemiology and global impact
CD affects mainly developed countries, with an annual incidence of up to 20.2 per 100 000 persons in North America, 12.7 in Europe and 5.0 in Asia and the Middle East (Molodecky et al., 2012). Systematic data reviews indicate that the incidence and prevalence of CD is increasing in both developed and developing nations, as the latter become more industrialized (Molodecky et al., 2012). Incidence rates are higher among the second to the fourth decade of life, which results in long-term costs to their productivity and the society (Yu et al., 2008; Molodecky et al., 2012). The extent of the contribution of AIEC to the onset and persistence of CD is still unclear and it has been proposed that CD is initiated by a dysregulated immunological response to normal intestinal micro-organisms, which creates an inflammatory milieu favoring expansion and invasion in a subset of patients (Strober, 2011). AIEC prevalence in CD patients varies in different studies from 22–52%, and a recent study found AIEC strains in 51.9% of ileal biopsies from CD patients, and only in 16.7% of healthy controls (Martinez-Medina et al., 2009). Another study found AIEC in 21.7% of chronic lesions, 36.4% in early lesions and 22.2% in healthy mucosa of CD patients, while only 6.2% of the mucosa was affected in controls (Darfeuille-Michaud et al., 2004). Thus, incidence rates suggest that AIEC strains could be contributing to CD in up to one-third of total cases of genetically susceptible individuals.
Molecular pathogenesis
Adherence and invasion
AIEC’s ability to adhere to epithelial cells is mediated primarily by the type I pili, which interact with the host receptor CEACAM6. This receptor is abnormally over-expressed by the ileal epithelium in CD patients (Carvalho et al., 2009). AIEC-induced colitis in transgenic mice depends on both bacterial type I pili expression and human CEACAM6 expression in the intestine (Carvalho et al., 2009). The earliest lesions of CD are microscopic erosions of the follicle-associated epithelia lining the Peyer’s patches (PPs). AIEC is able to adhere to the specialized M cells in PPs through the type I pili and the long polar fimbriae (Lpf) (Chassaing et al., 2011), favoring its translocation through M cells, which may explain the detection of AIEC in the lamina propria of CD patients. The invasion properties of AIEC are associated with the extrusion of microvillar extensions from the host cell membrane that engulf the bacteria, resembling macropinocytosis induced by other intracellular pathogenic bacteria (Darfeuille-Michaud, 2002). However, this mechanism for AIEC depends on both actin microfilaments and microtubules (Darfeuille-Michaud, 2002), while invasion for other pathogens depends exclusively on actin cytoskeleton rearrangements. Type I pili have been involved in the invasion of intestinal epithelial cells, and mutants in the fimI and fimF gene products, involved in the type I pili biogenesis, still adhere to IECs but their ability to induce membrane elongations at the site of contact is impaired (Boudeau et al., 2001). Further, outer membrane vesicles (OMV) containing biologically active proteins function as an efficient secretion pathway and are also important for AIEC invasion. For example, an yfgL mutant, showing reduced release of OMV, displays a diminished ability to invade intestinal cells (Rolhion et al., 2005). The endoplasmic reticulum stress protein Gp6 is also over-expressed in the ileum of CD patients; it co-localizes with CEACAM6 and functions as a receptor for the bacterial OmpA located on the surface of OMV (Rolhion et al., 2010). Although AIEC-specific content in the OMV is necessary for promoting invasion (Rolhion et al., 2010), the nature of the effector proteins delivered by OMV is still unknown.
Regulation
Compared to other E. coli pathotypes, little is known about the mechanisms regulating expression of virulence factors in AIEC. The expression of the fim operon is regulated by the orientation of an invertible DNA element that includes the fim promoter (the fim ON and OFF switch). The orientation of this invertible element is determined by the activity of the FimB and FimE recombinases (Klemm, 1986). In AIEC, fim is repressed in mutants defective in flagella assembly, generally as a result of a preferential switch towards the OFF state of the invertible element (Barnich et al., 2003, 2004; Claret et al., 2007). A reduction in the transcription of the fimB and fimE genes, together with a reduction in adherence and invasion, occurs in a strain defective in the NlpI lipoprotein (Barnich et al., 2004). The flagellar transcriptional regulator FlhD2C2 and the sigma factor FliA are required for the expression of type I pili, while the effect of FliA is partially mediated through YhjH, a phosphodiesterase involved in degradation of dimeric cGMP (Claret et al., 2007). While high osmolarity induces an increase in the ability of AIEC to interact with human cells and correlates with an increase in OmpC porin expression, an ompC null mutant expresses neither flagella nor type I pilli, and this effect is mediated by the RNA polymerase σE factor (Rolhion et al., 2007). In contrast, down-regulation of the histone-like protein Fis, occurring during AIEC infection of IECs, produces a repression of flagella expression and leads to the preferential ON status of the fim switch (Miquel et al., 2010a).
A Caenorhabditis elegans-based model for in vivo AIEC infection was developed and used to test whether Hfq RNA chaperone, which is involved in post-transcriptional regulation by small non-coding RNA, is required for full virulence. The hfq mutant is non-motile, less invasive and highly sensitive to chemical stress, indicating that post-transcriptional ribo-regulation mediates AIEC adaptation to this environmental niche (Simonsen et al., 2011). The transcription of the HtrA stress protein and the DsbA oxidoreductase, required for intramacrophage replication, are highly up-regulated in intramacrophagic bacteria and/or macrophage-mimicking stress culture conditions, but their activation is independent of the CpxRA two-component regulatory system (Bringer et al., 2005, 2007), which regulates HtrA and DsbA expression in non-pathogenic E. coli strains.
Avoidance of host responses
AIEC is able to translocate across in vitro cultured M cells monolayers (Chassaing et al., 2011). Enteric pathogens crossing the follicle-associated epithelia need to survive phagocytosis and bacterial killing by resident and recruited macrophages at the dome of the lymphoid follicle. Pathogenic bacteria counteract macrophage killing after phagocytosis, either by escaping phagosomes and inducing cell death, or by resisting the antimicrobial environment of the phagosome, which includes inhibition of its fusion with lysosomes. AIEC is able to survive and replicate within macrophages, in which they reside in a large vacuole without inducing cell death (Glasser et al., 2001). In contrast to other pathogens, AIEC-containing phagosomes traffic through the endocytic pathway and mature into active phagolysosomes where bacteria are exposed to low pH and the proteolytic activity of cathepsin D (Bringer et al., 2006). AIEC has developed mechanisms to resist phagosome stress conditions and mutations in htrA or dsbA increase the sensibility of the bacteria to acid and nutrient-limiting growth conditions and oxidative stress caused by hydrogen peroxide, which correlates with a defect in intramacrophagic replication (Bringer et al., 2005, 2007).
Putative genetic determinants predisposing humans to CD include polymorphisms of the gene encoding the intracellular receptor NOD2/CARD15 (Hugot et al., 2001; Ogura et al., 2001) and the genes ATG16L1 and IRGM, involved in intracellular bacterial clearance through autophagy (Massey and Parkes, 2007; Rioux et al., 2007). NOD2/CARD15 stimulation by bacterial muramyl dipeptide activates various antibacterial responses through NF-κB and MAP kinase signaling pathways (Fritz et al., 2006). The autophagy constitutes an efficient host innate immune mechanism against intracellular replication of CD-associated AIEC (Lapaquette et al., 2010, 2012). AIEC may take advantage of a diminished surveillance function of monocytes with NOD2/CARD15 polymorphisms, since monocytes expressing CD-associated alleles showed a reduced early cytokine response to AIEC infection (Peeters et al., 2007). Similarly, reducing the expression of ATG16L1 or IRGM in IECs abrogates autophagy of intracellular AIEC. This effect could not be reversed by the expression of a CD-associated ATG16L1 variant (Lapaquette et al., 2010). In agreement, impairing expression of NOD2/CARD15, ATG16L1, or IRGM in macrophages produces an increase in intramacrophagic AIEC (Massey and Parkes, 2007). Thus, it is hypothesized that dysfunctional immunological responses toward intracellular bacteria in susceptible individuals harboring CD-associated polymorphisms contribute to the persistence of AIEC inside macrophages (Lapaquette et al., 2012).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree