Box 35-1. Indications for the Initiation of ART in the Chronically HIV-1 Infected Patient
ART is recommended for all HIV-infected individuals. The strength of this recommendation varies depending on the pretreatment CD4 cell count:
■ CD4 cell count < 350 cells/mm3 (AI)
■ CD4 cell count 350–500 cells/mm3 (AII)
■ CD4 cell count > 500 cells/mm3 (BIII)
Regardless of CD4 cell count, initiation of ART is strongly recommended for individuals with the following conditions:
■ Pregnancy (AI)
■ History of an AIDS-defining illness (AI)
■ HIV-associated nephropathy (HIVAN) (AII)
■ HIV/hepatitis B virus (HBV) co-infection (AII)
Effective ART also has been shown to prevent transmission of HIV from an infected individual to a sexual partner; therefore, ART should be offered to patients who are at risk of transmitting HIV to sexual partners (AI: heterosexual transmission; AIII: other transmission risk groups)
Patients starting ART should be willing and able to commit to treatment and should understand the benefits and risks of therapy and the importance of adherence (AIII). Patients may choose to postpone therapy, and providers, on a case-by-case basis, may elect to defer therapy on the basis of clinical and/or psychosocial factors.
Rating of recommendations: A = strong; B = moderate; C = optional.
Rating of evidence: I = data from randomized controlled trials; II = data from well-designed nonrandomized trials or observational cohort studies with long-term clinical outcomes; III = expert opinion.
35-4. Drug Therapy
See Table 35-1 for antiretroviral agents recommended by the U.S. Department of Health and Human Services for initial treatment of established HIV infection.
Nucleoside Reverse Transcriptase Inhibitors
Nucleoside reverse transcriptase inhibitors (NRTIs) are described in Table 35-2. The mechanism of action of NRTIs is to interfere with HIV viral RNA (ribonucleic acid)-dependent deoxyribonucleic acid (DNA) polymerase, resulting in chain termination and inhibition of viral replication.
Didanosine (ddI), stavudine (d4T), and lamivudine (3TC) are dosed on the basis of weight. Most NRTIs are not affected by food (except didanosine). NRTIs have a low pill burden as a class and few drug interactions. All are pro-drugs requiring two or three phosphorylations for activation.
Four combination products are available:
■ Combivir (zidovudine 300 mg + lamivudine 150 mg) every 12 hours
■ Trizivir (zidovudine 300 mg + lamivudine 150 mg + abacavir 300 mg) every 12 hours
■ Truvada (tenofovir 300 mg + emtricitabine 200 mg) every 24 hours
■ Epzicom (lamivudine 300 mg + abacavir 600 mg) every 24 hours
No special storage requirements are necessary for drugs in this class.
Usually, two NRTIs are used in combination with one non-nucleoside reverse transcriptase inhibitor (NNRTI), one protease inhibitor (PI), or one integrase strand transfer inhibitor (INSTI).
All NRTIs have a boxed warning concerning the following class toxicities:
■ Lactic acidosis
■ Severe hepatomegaly with steatosis
The following precautions should be kept in mind regarding NRTIs:
■ Most patients should be dose adjusted for renal impairment (exception: abacavir).
■ Lamivudine and emtricitabine are chemically similar and should not be used in the same regimen.
■ Do not use zidovudine with stavudine because of antagonism (both require thymidine for activation).
Table 35-1. Antiretroviral Regimens for Treatment of HIV Infection in Antiretroviral-Naïve Patients
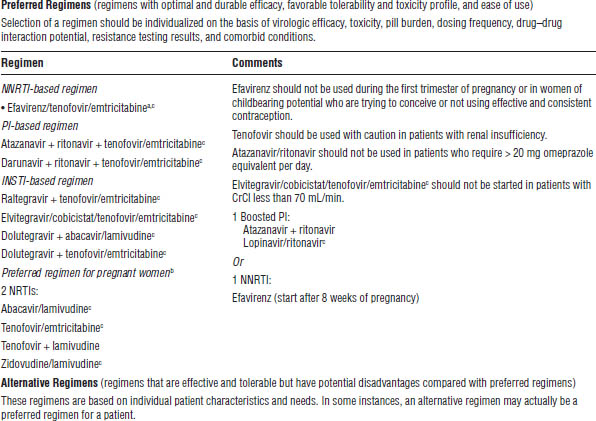
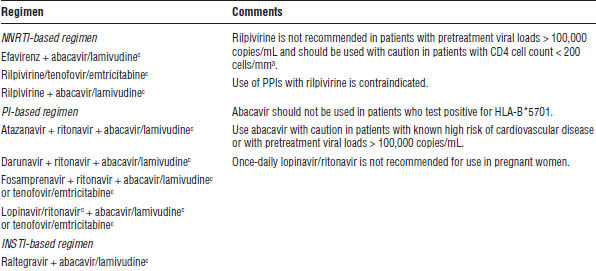
INSTI, integrase strand transfer inhibitor; PPI, proton pump inhibitor.
a. Lamivudine may be substituted for emtricitabine or vice versa.
b. Refer to the perinatal guidelines for more detailed recommendations (http://aidsinfo.nih.gov/guidelines).
c. The following are available as coformulated fixed-dose combinations: efavirenz/tenofovir/emtricitabine (Atripla), tenofovir/emtricitabine (Truvada), lopinavir/ritonavir (Kaletra), zidovudine/lamivudine (Combivir), abacavir/lamivudine (Epzicom), rilpivirine/tenofovir/emtricitabine (Complera), elvitegravir/cobicistat/tenofovir/emtricitabine (Stribild).
Table 35-2. Nucleoside Reverse Transcriptase Inhibitors
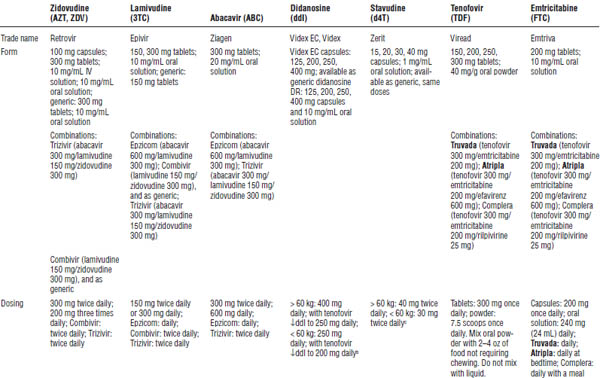
HBV, hepatitis B virus.
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
a. Monitor all for signs and symptoms of NRTI class toxicities, lactic acidosis, and hepatic steatosis; incidence is higher with stavudine than with other NRTIs.
b. Preferred dosing with oral solution is divided into two doses.
c. The World Health Organization recommends 30 mg twice daily regardless of body weight.
■ Do not use didanosine with stavudine during pregnancy because of increased risk of lactic acidosis and liver damage.
■ Tenofovir increases didanosine levels and decreases atazanavir levels. Dosage adjustments are required.
■ Patients should be tested for HLA-B*5701 to determine risk for hypersensitivity reaction to abacavir. Only patients testing negative should start abacavir.
■ The “D” drugs (ddI and d4T) can cause pancreatitis and peripheral neuropathy; when used together, this effect can be additive.
■ The “D” drugs are more closely associated with lactic acidosis.
Non-nucleoside Reverse Transcriptase Inhibitors
NNRTIs are described in Table 35-3. Their mechanism of action is to competitively inhibit reverse transcriptase, thereby resulting in inhibition of HIV replication.
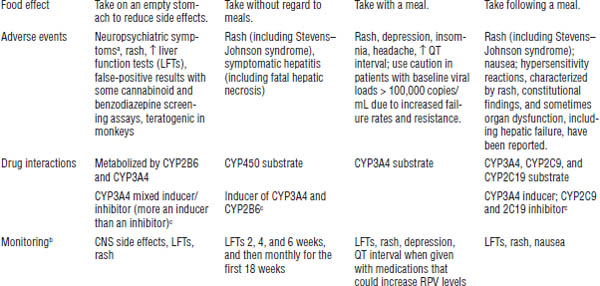
One-step mutation (K103N) confers resistance to first-generation NNRTIs (efavirenz, nevirapine, delavirdine) but not to the second-generation NNRTI etravirine (and rilpivirine in vitro). All should be dose adjusted for hepatic impairment.
Efavirenz should be taken on an empty stomach. Rilpivirine should be taken with the largest meal of the day. Efavirenz is contraindicated in pregnancy (pregnancy category D: risk of neural tube defects in first trimester).
Usually, one NNRTI is used in combination with two NRTIs. Two single-tablet regimens (STRs) include medications from this class:
■ Atripla (tenofovir 300 mg + emtricitabine 200 mg + efavirenz 600 mg) every 24 hours
■ Complera (tenofovir 300 mg + emtricitabine 200 mg + rilpivirine 25 mg) every 24 hours
No special storage requirements are necessary for drugs in this class. Class toxicities include rash and hepatic toxicity.
Drug interactions can occur (see Tables 35-4 and 35-5). All are cytochrome P450 (CYP) 3A4 inducers or inhibitors. Rilpivirine requires normal acid levels in the stomach for absorption. Class side effects include rash and elevations in liver enzymes.
Protease Inhibitors
Protease inhibitors are described in Table 35-6. Their mechanism of action is to inhibit protease, which then prevents the cleavage of HIV polyproteins and subsequently induces the formation of immature noninfectious viral particles.
Table 35-3. Non-nucleoside Reverse Transcriptase Inhibitors
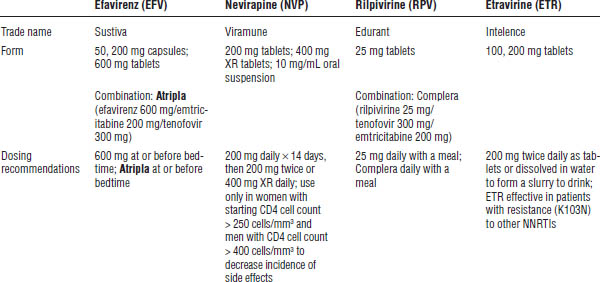
CNS, central nervous system.
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
a. CNS side effects include dizziness, somnolence, insomnia, abnormal dreams, confusion, abnormal thinking, impaired concentration, amnesia, agitation, depersonalization, hallucinations, and euphoria. Use caution in patients with a psychiatric history or previous addictions.
b. Monitor all for signs and symptoms of NNRTI class toxicities, rash, and hepatic toxicity.
c. See Tables 35-4 and 35-5.
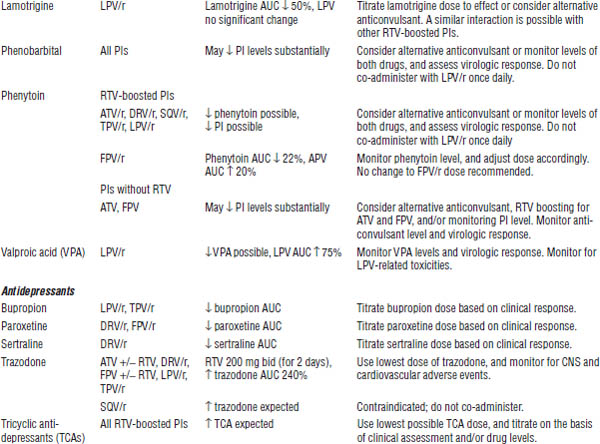
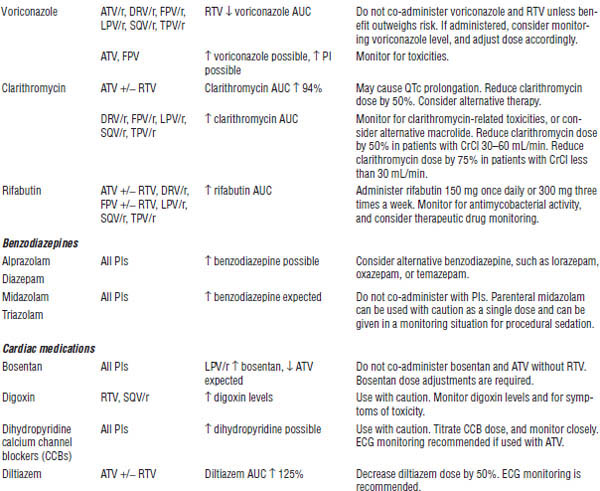
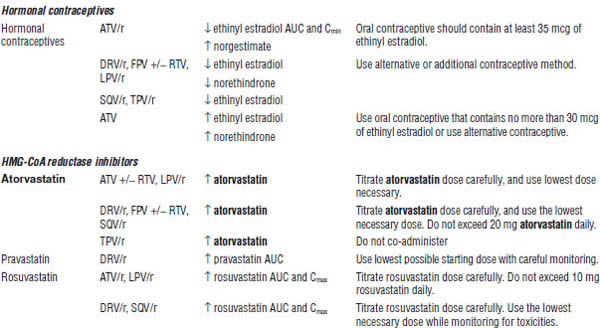
Table 35-4. Drugs That Should Not Be Used with NNRTIs
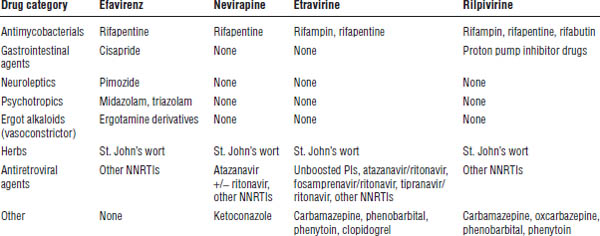
Table 35-5. Drug Interactions with NNRTIs Requiring Dose Modifications or Cautious Use
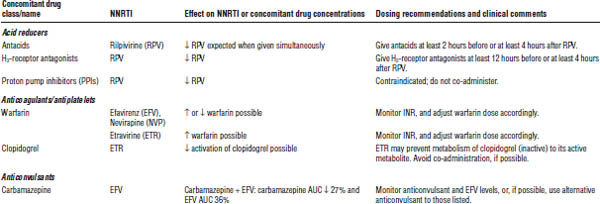
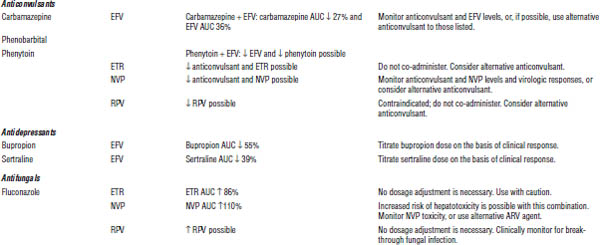
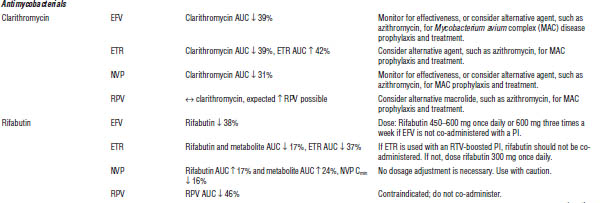

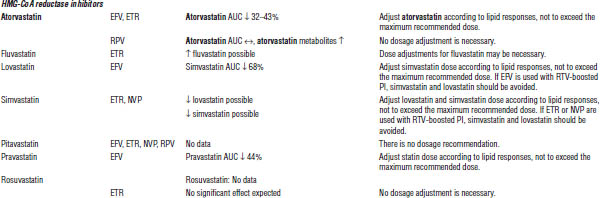
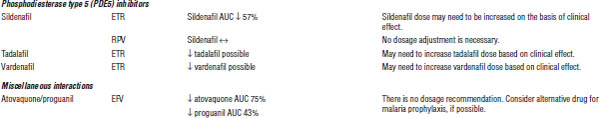
ARV, antiretroviral; AUC, area under the curve; HMG-CoA, 3-hydroxy-3-methyl-glutaryl-coenzyme A; INR, international normalized ratio; RTV, ritonavir.
Table 35-6. Protease Inhibitors
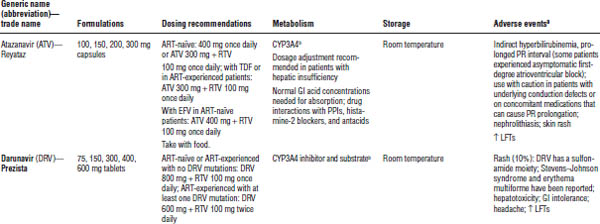



GI, gastrointestinal; LFT, liver function test; PPI, proton pump inhibitor.
a. Class side effects of all PIs: hyperlipidemia, particularly hypertriglyceridemia, hyperglycemia, fat maldistribution, possible increased bleeding episodes in patients with hemophilia.
b. See Tables 35-7 and 35-8.
All should be dose adjusted for hepatic impairment. Most should be taken with food (except fosamprenavir, tipranavir, lopinavir/ritonavir tablets, and indinavir). Atazanavir and indinavir require normal acid levels in the stomach for absorption and are associated with kidney stone formation and increases in indirect bilirubin. Darunavir, fosamprenavir, and tipranavir contain sulfa. Caution is warranted for patients with a history of severe sulfa allergies.
Most PIs are CYP3A4 inhibitors. Ritonavir is the most potent inhibitor in the class and is primarily used for intensification of other PIs. See Tables 35-7 and 35-8 for more information about drug interactions.
Ritonavir capsules, lopinavir/ritonavir solution, and tipranavir should be refrigerated.
Goals of intensification are as follows:
■ Decrease pill burden
■ Decrease frequency of doses (i.e., decrease from q8h to q12h)
■ Increase drug levels, resulting in decreased resistance
Class toxicities are as follows:
■ Fat maldistribution
■ Hyperglycemia
■ Hyperlipidemia
■ Possible increased bleeding episodes in patients with hemophilia
Baseline PI monitoring is done 4–6 weeks after starting the PI. Monitoring should then take place every 3–6 months thereafter. The following tests are required:
■ Glucose test
■ Liver function tests (LFTs)
■ Total cholesterol panel (particularly triglycerides)
■ Signs and symptoms of gastrointestinal (GI) side effects
■ Signs and symptoms of fat redistribution
Usually, one PI (boosted PIs preferred) is used in combination with two NRTIs.
Entry inhibitors
Entry inhibitors include enfuvirtide (T20) and maraviroc. Enfuvirtide is a fusion inhibitor, whereas maraviroc is a CCR5 (chemokine [C-C motif] receptor 5) antagonist. See Table 35-9 for information.
Enfuvirtide (T20) (Fuzeon)
Enfuvirtide’s mechanism of action is to bind to glycoprotein 41 on the HIV surface, thus inhibiting HIV binding to the CD4 cell.
The dose is 90 mg subcutaneous every 12 hours. Side effects include injection-site reactions, an increased rate of bacterial pneumonia, and hypersensitivity.
Enfuvirtide is generally reserved for deep salvage regimens. Preferably, it should be used with at least two other active drugs. Resistance develops quickly with less potent regimens and in cases of poor adherence.
No known significant drug interactions have been seen to date. Enfuvirtide can be taken without regard to meals. It should be stored at room temperature; the reconstituted form should be stored in the refrigerator, where it will be stable for 24 hours.
Maraviroc (Selzentry)
Maraviroc’s mechanism of action is to bind to CCR5 receptors on the CD4 cell surface, which inhibits HIV binding and entry into the CD4 cell.
Perform Trofile testing before using maraviroc to determine the patient’s tropism. The patient must be CCR5 tropic only.
Maraviroc is a CYP3A4 substrate. The dose depends on drug reactions:
■ Use 150 mg po every 12 hours when giving maraviroc with strong CYP3A4 inhibitors (most PIs).
■ Use 300 mg po every 12 hours when giving maraviroc with enfuvirtide, tipranavir/ritonavir, nevirapine, or weak CYP3A4 inhibitors.
■ Use 600 mg po every 12 hours when giving with CYP3A4 inducers (efavirenz, rifampin, etc.).
Side effects include abdominal pain, cough, dizziness, musculoskeletal symptoms, pyrexia, rash, upper respiratory tract infections, hepatotoxicity, and orthostatic hypotension.
Preferably, use maraviroc with at least two other active drugs. Take it without regard to meals.
Integrase strand transfer inhibitors
INSTIs include raltegravir (Isentress), dolutegravir (Tivicay), and elvitegravir (available only in combination tablet Stribild) (Table 35-10). INSTIs block activity of the integrase enzyme, thereby preventing HIV DNA from meshing with the CD4 cell DNA. INSTIs chelate with cations and should be separated from medications that contain them.
Table 35-7. Drugs That Should Not Be Used with PIs
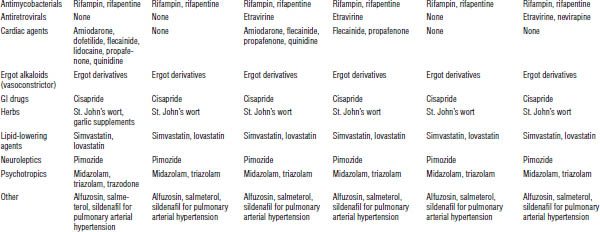
Table 35-8. Drug Interactions with PIs Requiring Dose Modifications or Cautious Use
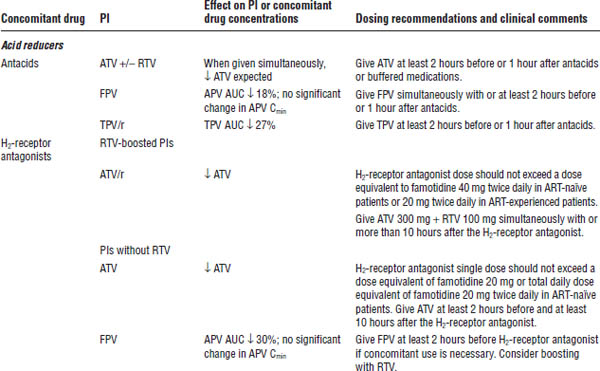
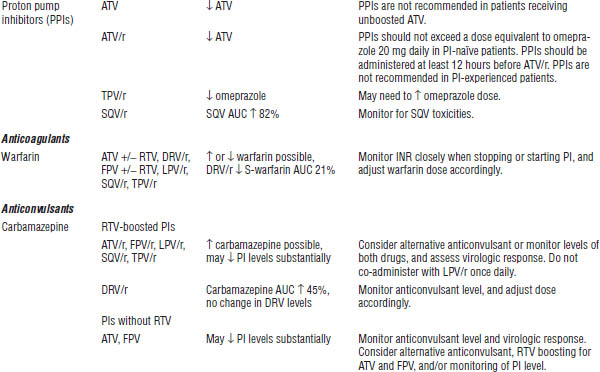
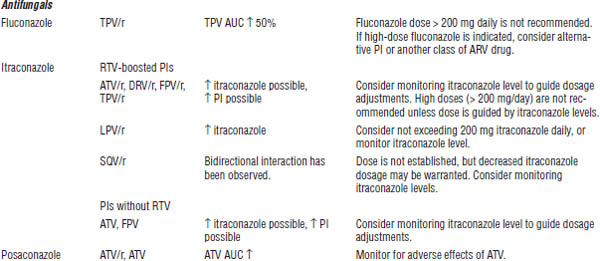
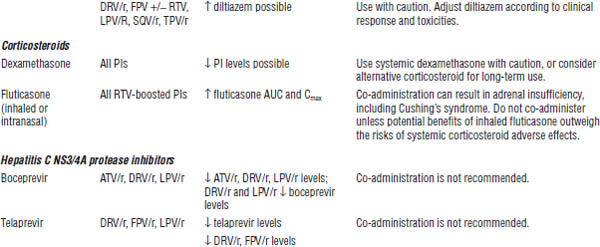
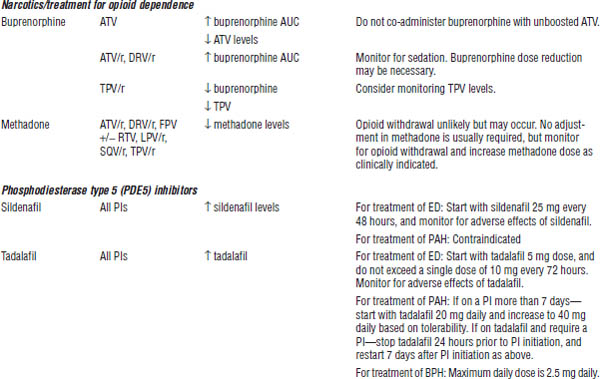
BPH, benign prostatic hyperplasia; CrCl, creatinine clearance; ECG, electrocardiogram; ED, erectile dysfunction; PAH, pulmonary arterial hypertension.
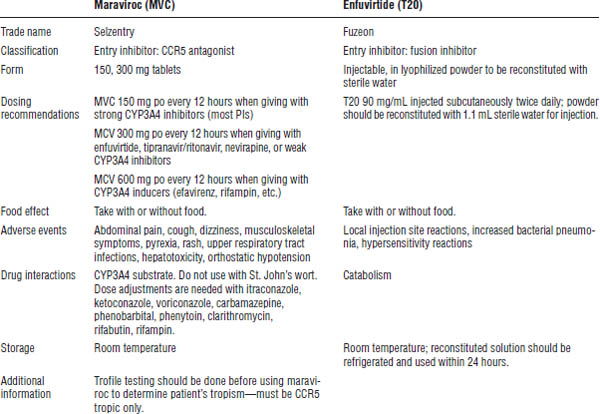
Table 35-10. Integrase Inhibitors
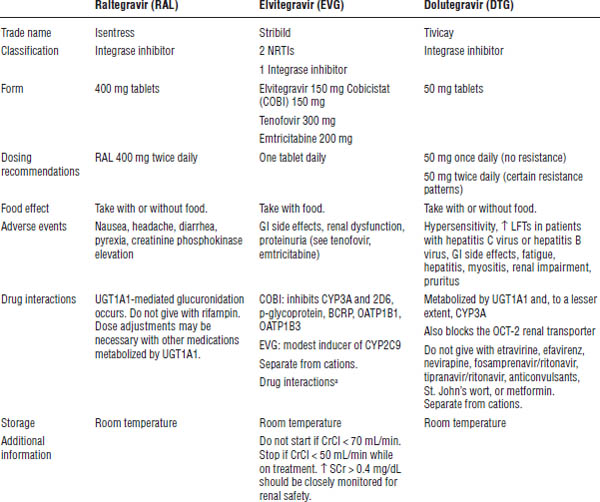
CrCl, creatinine clearance; SCr, serum creatinine.
a. See Table 35-7.
Raltegravir is metabolized through UDP-glucuronosyltransferase 1A1 (UGT1A1)-mediated glucuronidation. Drug interactions occur with rifampin and other drugs that affect UGT1A1. The dose is 400 mg po every 12 hours. Side effects include nausea, headache, diarrhea, pyrexia, and creatinine phosphokinase (CPK) elevation. Take it without regard to meals.
Dolutegravir is metabolized through UGT1A1 and, to a minor extent, CYP3A. It inhibits the OCT-2 renal transporter; therefore, mild elevations in serum creatinine may be seen. Drug interactions occur with metformin and other drugs that are eliminated through the OCT-2 renal transporter and with medications that are strong inhibitors or inducers of UGT1A1 (rifampin) or CYP3A (nevirapine, fosamprenavir/ritonavir, tipranavir/ritonavir, anticonvulsants).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree