10 How to use the monographs
General considerations
In this book, the monographs on individual herbs are designed to be as user-friendly as possible and hence are divided into two sections:
• A summary monograph which provides at a glance a definition, background material and clinically relevant information.
• A technical data section which extensively reviews the botany, pharmacology, clinical trial data, safety data and regulatory status in selected countries.
Common and botanical names
For example, the Echinacea species are currently being revised. A potential change includes E. angustifolia→E. pallida var. angustifolia.1 Changes which have been proposed, but will not be enacted due to possible detriment to the pharmaceutical, herbal and agricultural industries, include:2
With the use of gene-sequencing techniques, changes may increase in the future as a greater understanding of taxonomy at a genetic level develops. Of an estimated 5 million species (of living things), only about 1.5 million are documented at present and they are constantly being renamed and moved in the 20 or so categories of the Linnaean classification system. A new approach (phylogenetic nomenclature), which names groups of organisms that descend from a common ancestor is gaining popularity.3
• Mabberley DJ. The Plant Book, 2nd Edn. Cambridge University Press, Cambridge, 1997. In the preparation of this book the authors followed the system of Cronquist (1981) as modified by Kubitzki (1990) (pp. ix-xiii)
• PLANTS database, United States Department of Agriculture, USDA
• GRIN Taxonomy database, Agricultural Research Service, USDA
• Global Plant Checklist, International Organisation for Plant Information
The botanical name is usually a Latin binomial consisting of a generic name, which comes first and then the specific epithet. Both components of the name are italicised. The generic name, which is capitalised, defines the genus to which the plant belongs. The authority that follows the specific epithet further defines the species. It indicates the taxonomist credited with naming the species (and hence the author of the name) and is often abbreviated (e.g. ‘Linnaeus’ becomes ‘L’). The authority has been included in the initial identifying information in these monographs and if necessary in the Adulteration section, but is not retained throughout the remainder of the monograph.
Summary actions
The actions are important because they encompass the traditional pharmacology of herbal medicine and link the therapeutic requirements of the patient to the choice of herbs (see the Chapter 7). Only those actions which are considered to be well supported are listed.
Can be used for
This section effectively covers the clinical uses that can be recommended in practice.
Dosage
The authoritative texts used mainly comprise the following:
• British Herbal Medicine Association’s Scientific Committee. British Herbal Pharmacopoeia. BHMA, Bournemouth, 1983
• British Herbal Medicine Association’s Scientific Committee. British Herbal Pharmacopoeia, 4th Edn. BHMA, Bournemouth, 1996
• Pharmacopoeia Commission of the People’s Republic of China. Pharmacopoeia of the People’s Republic of China, English Edn. Chemical Industry Press, Beijing, 1997
• Bensky D, Gamble A. Chinese Herbal Medicine Materia Medica. Eastland Press, Seattle, 1986
• Scientific Committee of ESCOP (European Scientific Cooperative on Phytotherapy). ESCOP Monographs. European Scientific Cooperative on Phytotherapy, ESCOP Secretariat, UK, March 1996–October 1999
• Blumenthal M, et al (eds). The Complete German Commission E Monographs: Therapeutic Guide to Herbal Medicines. American Botanical Council, Austin, 1998.
• Kapoor LD. CRC Handbook of Ayurvedic Medicinal Plants. CRC Press, Boca Raton, 1990
• Regional Research Laboratory and Indian Drug Manufacturers’ Association. Indian Herbal Pharmacopoeia. Indian Drug Manufacturers’ Association, Mumbai and Regional Research Laboratory, Jammu-Tawi, 1998
In addition, dosages used in clinical trials were also taken into account.
Summary assessment of safety
This section summarises the safety data detailed in the Technical data section.
Technical data
Adulteration
CITES (the Convention on International Trade in Endangered Species of Wild Fauna and Flora) currently provides the only international instrument for the listing of species considered to be sufficiently endangered to the extent that commercial trade must be either monitored and controlled or prohibited.4
Pharmacodynamics
When extrapolating from animal studies a common misconception is that the dose used in the animal (mg/kg) directly relates to the human dose on a body weight basis. In other words, the mg/kg dose in the animal is to be multiplied by the average human weight of 70 kg to give the corresponding human dose in mg. Such considerations are important in toxicology and new drug development (where an effective human dose needs to be worked out from prior animal studies). But animals have much faster metabolism, so a correction factor needs to be applied. One publication has defined the scale-up factors for common animal models. This is around 6 for the mouse and 11 for the rat.5 In other words if a rat study used 100 mg/kg of extract, the corresponding human dose (for 70 kg) is 1.135 g, not 7.0 g. This can only apply if oral doses were used in the animal model. Other assumptions are that the model is relevant to the human disease and the animal metabolises the agent in the same way as humans.
Pharmacokinetics
The known pharmacokinetics of key constituents is reviewed, giving emphasis to human studies.
Clinical trials
Emphasis is given to randomised, controlled, double blind clinical trials. However, open (not blinded) and uncontrolled (no control group) trials are also briefly summarised. Where a number of clinical trials have been subjected to meta-analysis, or systematic review, this publication is primarily reviewed rather than those individual trials included in the meta-analysis or review, although important individual studies may also be highlighted. Also see Appendix E.
Relevance of scientific studies to clinical choices
• In vivo, herb given by injection: no relevance unless known active components have established bioavailability, even then of limited value
• In vivo, herb given orally: some relevance, depending on dose and validity of the animal model
• Clinical study: highly relevant, but depending on the design of the trial and being able to reproduce the treatment used.
Toxicology and other safety data
Toxicology
Abbreviations used throughout this section:
| ig* | intragastric |
| im | intramuscular |
| ip | intraperitoneal |
| iv | intravenous |
| LD50 | lethal dose for 50% of the tested population |
| LOAEL | lowest observed adverse effects level |
| MLD | minimum lethal dose |
| The least amount of a chemical that can produce death | |
| MOAEL | minimum observed adverse effect level |
| MTD | maximum tolerated dose |
| NOAEL | no observed adverse effect level |
| The highest dosage administered that does not produce toxic effects | |
| Sc | subcutaneous |
| TLV | threshold limit value |
| TTL | threshold toxic limit |
* In some cases ‘oral’ has been used for ‘gavage’ and ‘ig’ administration.
LD50 test
The LD50 test was introduced in 1927 for the biological standardisation of drugs.6 With the mean lethal dose (LD50) test, groups of experimental animals are treated with graduated doses of a test substance with the aim of obtaining a 50% or even higher mortality at the highest doses. The scientific significance of the classical LD50 test has been questioned on the basis of the relatively broad variability of the test results (more than 2-fold and up to 11-fold differences) and for animal welfare reasons.7 Three recently developed alternative animal tests that significantly improve animal welfare – the fixed dose procedure, the acute toxic class method, and the up and down procedure – can now be used within a strategy of acute toxicity testing for all types of test substances and for regulatory and in-house purposes. In vitro cytotoxicity tests could be used as adjuncts to these alternative animal tests to improve dose level selection and reduce (at least modestly) the number of animals used. However, the total replacement of animal tests requires a considerable amount of further development8 and such modern data are not yet currently available for most herbs.
The LD50 values can be grouped into toxicity levels,9 as outlined in the following table with examples.
| Lethal dose | Toxicity level | Example(s) |
|---|---|---|
| <1 mg/kg | Dangerously toxic | Dioxin – 0.045 mg/kg (oral, rat (female)) |
| 1–50 mg/kg | Extremely toxic | Indomethacin – 12.6 mg/kg (oral, rat) |
| Dieldrin – 46 mg/kg (oral, rat) | ||
| 50–500 mg/kg | Very toxic | Aristolochic acid – 55.9 mg/kg (oral, mouse (male)) |
| Curare – 270 mg/kg (oral, rabbit) | ||
| Paracetamol – 338 mg/kg (oral, mouse) | ||
| Caffeine – 355 mg/kg (oral, rat (male)) | ||
| 500–5000 mg/kg | Moderately toxic | Atropine – 622 mg/kg (oral, rat) |
| Aspirin – 1500 mg/kg (oral, rat) | ||
| Baking soda – 4220 mg/kg (oral, rat) | ||
| 5000–15 000 mg/kg | Slightly toxic | Sodium cyanide – 6444 mg/kg (oral, rat) |
| Monosodium succinate (food additive) –>8 g/kg (oral, rat) | ||
| >15000 mg/kg | Practically non-toxic | Propylene glycol (cosmetics) – 20 000 mg/kg (oral, rat) |
Ames salmonella/microsome mutagenicity assay (Salmonella test, Ames test)
This is a short-term bacterial reverse mutation assay specifically designed to detect a wide range of chemical substances producing genetic damage leading to gene mutations.10 The test was developed by Ames and colleagues in the mid-1970s and became the most used test because of its initial promise of high qualitative (yes/no) predictivity for cancer in rodents and, by extension, in humans. The relationship between mutagenic potency prediction and quantitative carcinogenicity, however, is now known to be weak,11 despite the fact that early studies with this assay indicated that greater than 90% of the known carcinogens tested were mutagenic and that 90% of the non-carcinogens tested were non-mutagenic. The power of this assay was derived from the use of a liver microsome fraction (S9 mix) containing the mixed function oxidase (cytochrome P450) enzymes required to activate the test substance into precarcinogens (as might occur in the body after phase I metabolism by the liver). As the basis of the selection of chemicals for mutagenicity testing shifted to relative environmental importance, the sensitivity of the Salmonella assay for detecting carcinogens decreased. A negative result does not imply that the chemical will be non-carcinogenic. There are a large number of false-negatives produced (i.e. non-genotoxic carcinogens).12 Some plant components such as flavonoids give a false positive on this test.
Use in pregnancy and lactation
(The pregnancy categories are adapted from the Australian publication Medicines in Pregnancy, 4th Edn, 1999.)
| Category A | No proven increase in the frequency of malformation or other harmful effects on the fetus despite consumption by a large number of women. |
| Category B1 | No increase in frequency of malformation or other harmful effects on the fetus from limited use in women. No evidence of increased fetal damage in animal studies. |
| Category B2 | No increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Animal studies are lacking. |
| Category B3 | No increase in frequency of malformation or other harmful effects on the fetus from limited use in women. Evidence of increased fetal damage in animal studies exists, although the relevance to humans is unknown. |
| Category C | Has caused or is associated with a substantial risk of causing harmful effects on the fetus or neonate without causing malformations. |
| Category D | Has caused or is associated with a substantial risk of causing fetal malformation or irreversible damage. |
| Category X | High risk of damage to the fetus. |
Regulatory status in selected countries
The regulatory status of the herb in Australia, China, Germany, the UK and the USA is presented.
Australia
Part 4 of Schedule 4 of the Therapeutic Goods Regulations specifies herbs which cannot be contained in any products listed on the Australian Register of Therapeutic Goods (ARTG), or which can only be present in minute doses or if other specified conditions are met. In other words, herbs in Part 4 of Schedule 4 are considered to be more toxic and cannot be included in over-the-counter herbal products without further safety evaluations (see below).
References
1. Binns SE, Baum BR, Arnason JT. Syst Bot. 2002;27(3):610–632.
2. Binns SE, Baum BR, Arnason JT. Taxon. 2001;50(4):1199–1200.
3. Milius S. Sci News. 1999;156(17):268–270.
4. Lewington A. Traffic Int. 1993:31–32.
5. Reagan-Shaw S, Nihal M, Ahmad N. FASEB J. 2008;22(3):659–661.
6. DePass LR. Toxicol Lett. 1989;49(2-3):159–170.
7. Schlede E, Mischke U, Roll R, et al. Arch Toxicol. 1992;66:455–470.
8. Botham PA. ILAR J. 2002;43(suppl):S27–S30.
10. Mortelmans K, Zeiger E. Mutat Res. 2000;455(2):29–60.
11. Fetterman BA, Kim BS, Margolin BH, et al. Environ Mol Mutagen. 1997;29(3):312–322.
Andrographis
Synonyms
Chiretta, King of bitters (Engl), kalmegh (Bengali, Hindi), kirata (Sanskrit), chuan xin lian (Chin), senshinren (Jap).
Traditional view
In Ayurvedic medicine, the herb is used for its bitter tonic, stomachic, antipyretic and laxative properties. It is said to increase appetite, strengthen digestion and diminish flatulence, hyperacidity and biliousness.1 The herb is also utilised for treatment of many other conditions, including diabetes, debility and hepatitis.2 The roots and leaves have a reputation for being depurative and anthelmintic.3 In traditional Chinese medicine, Andrographis is bitter and ‘cold’, and is used to clear Heat from the Blood (especially in the lungs, throat and urinary tract) and to detoxify Fire Poison (manifesting as skin sores and carbuncles). In addition to gastrointestinal complaints, it is prescribed for throat infections, cough with thick sputum and snake bites.4,5 Since Andrographis is regarded as a ‘cold’ herb, it is ideally suited to treating acute infections, which are ‘hot’ conditions.
Dosage
Being very bitter, some people may find Andrographis difficult to take in liquid preparations. Whichever way it is taken, the daily preventative dose for an adult is about 2 to 3 g or its equivalent (for example, 4 to 6 mL/day of a 1:2 fluid extract). During infection, the effective dose is nearer to 6 g/day (up to 12 mL/day of the 1:2). Standardisation for andrographolides is preferable.
Technical data
Botany
Andrographis paniculata is an annual shrub belonging to the Acanthaceae family. It grows to a height of 1 metre with branches that are sharply quadrangular, often narrowly winged towards the apical region. The leaves are lanceolate (5 to 8 cm long), and the flowers are small, solitary in panicles, with a corolla ranging from white to rose pink in colour and hairy externally. The fruit is approximately 2 cm long, linear-oblong in shape and acute at both ends. The seeds are numerous, yellowish-brown and glabrous.6 It grows wild as an undershrub in tropical moist deciduous forests6 and is also cultivated as a rainy season crop.2
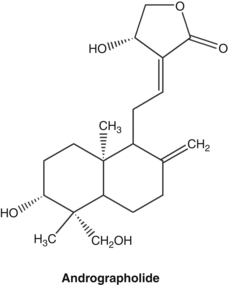
Key constituents
• Diterpenoid lactones, collectively referred to as andrographolides, and consisting of aglycones (such as andrographolide) and glucosides (such as neoandrographolide and andrographiside)7,8
• Diterpene dimers,7 flavonoids9
Many of the diterpenoid lactones (such as andrographolide) are bitter; however, neoandrographolide is not.8
Adulteration
Andrographis echioides is an adulterant of A. paniculata.11 Purchased extracts of Andrographis paniculata are sometimes devoid of any andrographolide content, despite claims to the contrary on product specifications.12
Pharmacodynamics
Anti-infective and immunomodulating activity
Although Andrographis is widely used in infections and infestations, the most likely opinion is that its value here is mainly as an immune-enhancing treatment. Early reports in China attributed an antibacterial activity to the plant that was not supported in a later review.5 No direct antibacterial activity could be demonstrated for an aqueous extract of Andrographis against Salmonella, Shigella, Escherichia coli, group A streptococci and Staphylococcus aureus in vitro. Animal studies using orally administered Andrographis (0.12 to 0.24 g/kg) for 6 months failed to demonstrate bactericidal activity.13 Serum taken from 10 healthy volunteers after a single oral dose of Andrographis (ranging from 1 g to 6 g) showed no bactericidal activity against a number of organisms.13
However, an alcoholic extract of Andrographis did show significant activity against an E. coli enterotoxin-induced secretory response (that causes diarrhoea) in vivo,14 and in another study effected in vitro inhibition of adherence of Streptococcus mutans.15 Andrographolide potentiated the sensitivity of two strains of Pseudomonas aeruginosa to several antibiotic drugs in vitro.16 Significant growth inhibition (compared to five other herbs) was recently demonstrated for an Andrographis aqueous extract in vitro against Streptococcus agalactiae.17 Feeding Nile tilapia fish (Oreochromis niloticus) with Andrographis reduced mortality following infection with this species of Streptococcus (although such an outcome could also reflect enhanced immunity).
Liquid extract of Andrographis root demonstrated strong in vitro anthelmintic activity against human Ascaris lumbricoides.18 Subcutaneous administration of a decoction of Andrographis leaves to infected dogs reduced nematode larvae in the blood by 85%.19
Early uses as a substitute for quinine in malaria have been supported by recent research. An in vitro study revealed that xanthones from Andrographis root bearing a hydroxyl group at position 2 demonstrated the most potent antimalarial activity, while xanthones with a hydroxyl group at position 1, 4 or 8 possessed very low activity. Further, in vivo antimalarial testing of the most active xanthone on Swiss Albino mice with Plasmodium berghei infection demonstrated a substantial reduction (62%) in parasitaemia after treatment with 30 mg/kg.10 However, more relevant to current herbal use are studies on Andrographis leaf or whole plant. An earlier study showed that a chloroform extract of the whole plant demonstrated complete parasite growth inhibition within a 24 hour incubation period in vitro at concentrations as low as 0.05 mg/mL. There was significant reduction in mortality rates in mice administered Andrographis whole plant extract (5 mg/kg/day, ip for 4 days) just after malarial infection.20 Methanolic extracts of Andrographis leaves21 and aerial parts22 were active in vitro against both chloroquine resistant and sensitive strains of Plasmodium falciparum.
Dehydroandrographolide succinic acid monoester (DASM), a drug derived from andrographolide, has been found to inhibit HIV in vitro. This effect was observed on several HIV strains and DASM was non-toxic to other cells in the concentration range. However, the diterpenoid lactones of Andrographis (dehydroandrographolide and andrographolide) were devoid of anti-HIV activity.23 Moreover, in vitro studies with aqueous extracts of Andrographis showed little or no inhibition of HIV-1. Modes of inhibition studied comprised inhibition of HIV-1 protease,24 inhibition of the interaction between HIV-1 gp 120 and immobilised CD4 receptor, inhibition of HIV-1 reverse transcriptase and inhibition of glycohydrolase enzymes.25
Andrographolides showed in vitro activity against herpes simplex virus 1 in vitro26 and both Andrographis ethanolic extract (25 μg/mL) and andrographolide (5 μg/mL) inhibited the expression of Epstein-Barr virus lytic proteins in vitro, thereby inhibiting viral maturation.27 Andrographolide showed significant activity against influenza A viruses in vitro, including the H5N1 strain.28 Administration of andrographolide (100 to 200 mg/kg/day, oral) to mice infected with avian influenza A strains H9N2 and H5N1 and the human strain H1N1 significantly reduced death rate, prolonged life, inhibited lung consolidation and reduced viral titres in the lung.28 However, such in vivo activity might also be the consequence of immune effects.
Early research suggested an immunostimulant action for Andrographis. Enhanced phagocytosis was demonstrated in vitro for a decoction of the herb and in vivo after injection of the soluble derivatives.5 Isolated andrographolide (4 mg/kg/day, ip for 2 days) and an ethanolic Andrographis extract (25 mg/kg/day, oral for 7 days) significantly stimulated both antigen-specific and innate immune responses in mice. The whole extract produced stronger immunostimulation.29 Prolonged survival in animals after snakebite was observed after pretreatment with extracts of Andrographis.30
Four later studies (three from the same research group) have demonstrated a downregulation of immune response by andrographolide using both in vitro and in vivo models. In vitro, andrographolide reduced T cell activation in splenocytes,31 reduced IL-2 production in stimulated Jurkat T cells by interfering with nuclear factor of activated T cells (NFAT) and mitogen-activated protein kinases (MAPK),32 reduced IL-2 and interferon-gamma in stimulated murine T cells,33 and downregulated macrophage immune responses and cytokine expression.34 In vivo, andrographolide (4 mg/kg/day, ip) reduced T cell function and significantly reduced the severity of experimental autoimmune encephalomyelitis in mice, including antimyelin T cell and antibody responses.31 Andrographolide (1 mg/kg/day for 7 days, ip) also reduced antibody production and the number of IL-4-producing splenocytes in mice after antigen challenge.34
However, several other later publications have observed an enhanced immune response from andrographolide or Andrographis extract, most notably the in vivo studies summarised in the Antitumour activity section that follows. In addition, andrographolide and a combination of Andrographis and Siberian ginseng extracts (Kan Jang) demonstrated lymphocyte proliferation and stimulation of some cytokines in vitro in a whole blood cell culture.35 Andrographis extract (25 or 50 mg/kg/day, oral for 14 or 28 days) and andrographolide (1 or 4 mg/kg/day, oral for 14 or 28 days) enhanced specific antibody and cell-mediated immune responses in mice inoculated with an inactivated Salmonella vaccine.36 A mixture of andrographolides (1.0, 1.5 and 2.5 mg/kg, oral) potentiated delayed-type hypersensitivity (DTH) in mice inoculated with sheep red blood cells, but also countered the increase in DTH after cyclophosphamide treatment.37 This suggests an immunomodulatory activity. Further experimentation at the same doses revealed that the andrographolide mixture significantly stimulated phagocytic activity, white blood cell counts and spleen and thymus weights in mice.
Antitumour activity
A methanol extract of Andrographis showed potent cell differentiation-inducing activity on mouse leukaemia cells in vitro. Some of the isolated diterpenes also demonstrated this activity.7,38 Andrographolide was shown to inhibit the in vitro proliferation of more than 30 tumour cell lines representing various types of cancers, specifically breast, CNS, colon, lung, melanoma, ovarian, prostate, renal and leukaemia. The compound was found to exert direct anticancer activity on cancer cells by cell-cycle arrest via induction of p27 and decreased expression of cyclin-dependent kinase 4 (CDK4). It also enhanced tumour necrosis factor-alpha (TNF-alpha) in lymphocytes, and possibly via this mechanism increased their cytotoxic activity against cancer cells in vitro.39 Andrographolide (100 and 200 mg/kg/day for 10 days, oral) significantly inhibited the growth of B16 melanoma and HT-29 colon tumours in mice.39
Since these publications there has been a considerable number of in vitro investigations of the antineoplastic activity of andrographolide against a wide variety of cancer cell lines. An extensive review of material up to 2008 by Varma and co-workers identified the key mechanisms involved.40 These included induction of cell-cycle arrest (possibly due to increased levels of p21) and apoptosis (via a variety of mechanisms involving caspase-3, caspase-8, BcL-2 and TNF-alpha-related apoptosis inducing ligand). Other mechanisms have been proposed from recent research, including a downregulation of epidermal growth factor receptors,41 a decrease of cell-cycle related proteins,42 changes in the intracellular redox system43 and a novel cell differentiating activity.44 Other andrographolides have also been shown to induce cell-cycle arrest in vitro.45
Neoandrographolide sensitised the cytotoxic action of etopiside against a leukaemia cell line46 and andrographolide was found to sensitise cancer cells (such as colorectal, cervical and hepatic) to doxorubicin.47
Other researchers have investigated inhibition of angiogenesis as an anticancer prospect for Andrographis extract and andrographolide. Intraperitoneal administration of both in angiogenesis-induced mice led to substantial reductions in elevated proinflammatory cytokines such as IL-1beta, IL-6, TNF-alpha and granulocyte-macrophage colony-stimulating factor (GM-CSF) and the most potent angiogenic factor, vascular endothelial growth factor (VEGF). Antiangiogenic factors such as tissue inhibitor of metalloproteinase 1 (TIMP-1) and IL-2 levels were elevated after treatment.48 The inhibitory effect on VEGF production was supported by a later in vitro study.49
Andrographolide inhibited the adhesion of gastric cancer cells to endothelial cells in vitro by blocking E-selectin expression in the latter.50 Taiwanese scientists have found inhibition of migration and invasion of cancer cell lines in vitro via the downregulation of matrix metalloproteinase-7 (MMP-7)51,52 and MMP-2.53
There have also been additional in vivo anticancer studies on andrographolide and Andrographis extract (both administered intraperitoneally). A 70% ethanolic extract of Andrographis (10 mg/animal for 10 days, ip) substantially reduced tumour growth, helped maintain total white cell count, improved IL-2 and GM-CSF levels and reduced TNF-alpha in mice inoculated with Dalton’s lymphoma ascites cells.54 These effects were maintained for 11 to 20 days after the final herb dose and were also observed for animals concurrently treated with a combination of cyclophosphamide, radiation and whole body hyperthermia. The same authors also observed that both Andrographis extract (10 mg/animal for 10 days, ip) and andrographolide (0.5 mg/animal for 10 days, ip) substantially prolonged the survival times of mice inoculated with EL4 thymoma cells.55 IL-2 and interferon-gamma levels were increased and the authors concluded, based on this and a series of complex experiments, that the two treatments increased cytotoxic T lymphocyte activity. In general, the Andrographis extract was more active than andrographolide. The same research group using similar models and treatments has also demonstrated enhanced natural killer cell activity and antibody-dependent cytotoxicity in normal and tumour-bearing (Ehrlich ascites carcinoma) mice.56
Hepatoprotective and choleretic activity
Andrographolide showed protective activity against chemically induced toxicity in rat hepatocytes in vitro. The observed hepatoprotective effect was greater than silymarin.57 Intraperitoneal administration of andrographolide, andrographiside and neoandrographolide (100 mg/kg) to mice protected against hepatotoxic damage caused by carbon tetrachloride and tert-butylhydroperoxide. Andrographiside and neoandrographolide had the greatest effect on reducing lipid peroxidation and were comparable to silymarin.58 Similar studies suggest that andrographolide is the major active antihepatotoxic principle in Andrographis.59 Intraperitoneal administration of three diterpene constituents of Andrographis showed protective effects on hepatotoxicity induced in mice by various chemicals. The protective effect of andrographiside and neoandrographolide was as strong as silymarin, and could be due to the glucoside groups acting as strong antioxidants.60 Andrographolide exhibited hepatoprotective activity after oral or intraperitoneal administration to rats with chemically induced acute hepatitis. Treatment with the herb led to complete normalisation of five biochemical parameters and improved histopathological changes in the liver.61
Intraperitoneal pretreatment of mice with different doses of andrographolide or arabinogalactan proteins from Andrographis for 7 days was followed by intraperitoneal injection of ethanol (7.5 g/kg of body weight). At 500 mg/kg and 125 mg/kg, respectively, the protective activity of these two preparations against hepatic and renal alcohol toxicity was comparable to silymarin.62
Oral administration of Andrographis extract and andrographolide to rats demonstrated a protective action against carbon tetrachloride-induced hepatotoxicity. The leaf extract showed stronger activity than andrographolide.63 Pre- and post-treatment with oral doses of Andrographis (0.5 g/kg/day) normalised alcohol-induced increases in serum transaminase activity in rats. The researchers concluded that Andrographis has a protective as well as a curative effect on alcohol-induced toxic liver damage.64
Andrographolide (5, 7 and 10 mg/kg, oral) improved levels of antioxidant parameters such as glutathione, superoxide dismutase and catalase in mice treated with the liver carcinogen hexachlorocyclohexane (BHC).65 It also reduced parameters of liver damage and the development of liver tumours in BHC-treated mice at the same doses.66
Significant hepatoprotective activity was demonstrated for an alcohol extract of Andrographis and two of its diterpenes – andrographolide and neoandrographolide – against the hepatotoxicity caused by Plasmodium berghei infection in animals. The protective effect of Andrographis was thought to be partially due to reactivation of superoxide dismutase, which in turn counteracted peroxidative damage caused by the infection. Andrographis may also induce drug metabolising systems that detoxify hepatotoxins.67 Administration of Andrographis (0.5 g/kg/day) or andrographolide (5.0 mg/kg/day) to rats for 7 to 30 consecutive days induced the liver microsomal drug-metabolising enzymes aniline hydroxylase, N-demethylase and O-demethylase.68
Andrographolide produced a dose-dependent choleretic effect (increased bile flow, bile salt and bile acids) in rats and guinea pigs after oral administration69 and by intraperitoneal injection in rats.70 The effect was stronger than silymarin.69 Aqueous extract of Andrographis orally administered to rats at 3.75 mL/kg increased bile flow and liver weight. A maximal increase in flow and weight was reached after 2 days.71
Cardiovascular activity
An early study investigating an aqueous extract by intravenous administration suggested that Andrographis may limit the expansion of the ischaemic focus, may exert a marked protective effect on the reversible ischaemic myocardium and could demonstrate a weak fibrinolytic action.72 Andrographis alleviated myocardial ischaemia-reperfusion injury in vivo.73 It upregulated cellular reduced glutathione and protected cardiomyocytes against hypoxia/reoxygenation injury in vitro.74 The mechanism was probably via a decrease in the harmful effect of oxygen free radicals.75 A study using rabbits found Andrographis alleviated atherosclerotic arterial stenosis induced by both de-endothelialisation and a high cholesterol diet. In addition, it lowered the restenosis rate after experimental angioplasty.76 Andrographolide (5 mg/kg, presumably ip) suppressed the hyperplasia of arterial neointima (about a 60% reduction) in a murine model of arterial restenosis. This was via the downregulation of NF-kappaB target genes that are critical in thrombosis and inflammation.77
An aqueous extract of Andrographis given by intraperitoneal infusion to rats exhibited a dose-dependent reduction in systolic blood pressure in spontaneously hypertensive rats and normotensive controls.78 A crude water extract of Andrographis, and two semi-purified n-butanol and aqueous fractions, significantly reduced mean arterial blood pressure in anaesthetised rats without decreasing heart rate after ip administration. The hypotensive substance in the crude water extract appeared to be concentrated in the butanol fraction.79
Following the observation that some patients exhibited a hypotensive response while taking Andrographis, the in vitro and in vivo actions of the herb and three of its diterpenoids were investigated.80 The diterpenoid 14-deoxy-11,12-didehydroandrographolide (DIAP) was most active at reducing the chronotropic response of isolated rat atria and exerting spasmolytic activity in rat aortic rings. An Andrographis aqueous extract with the highest levels of DIAP (delivering 19 mg/kg of this compound, oral doses for 7 days) was the most potent (compared to two other extracts with lower levels of DIAP) at reducing systolic blood pressure in rats. Mechanistic studies suggested that vascular smooth muscle was the major site of the hypotensive effect.
Andrographolide inhibited PAF-induced human platelet aggregation81 and deoxyandrographolide antagonised PAF-mediated processes in neutrophils,82 as did andrographolide,83 all in vitro.
Anti-inflammatory, antipyretic, antiallergic and analgesic activity
Several early in vivo studies found antipyretic and anti-inflammatory effects for andrographolides (after oral administration or injection). The anti-inflammatory activity of the andrographolides may be due to the promotion of ACTH (adrenocorticotrophic hormone) and consequent enhancement of adrenocortical function.5 Andrographolide administered orally (30, 100 and 300 mg/kg) significantly reduced inflammation in a number of animal models including adjuvant-induced arthritis.84 The addition of andrographolide to an endothelial cell culture together with TNF-alpha caused a concentration-dependent reduction of the enhancement of endothelial monocyte adhesion, which is part of the inflammatory process.85 Another in vitro study found that andrographolide inhibited NF-kappaB binding to DNA, thereby reducing the expression of pro-inflammatory proteins such as COX-2 in neutrophils.83 Andrographolide prevented oxygen radical production by human neutrophils in vitro.86
As well as demonstrating antioxidant activity in vitro and in vivo, a 70% methanolic extract of Andrographis (10 mg/animal for 5 days, ip) completely inhibited carrageenan-induced paw oedema in mice.87 Oral administration of neoandrographolide (150 mg/kg) also demonstrated anti-inflammatory activity in mice as well as in vitro, using several experimental models.88
In a murine model of asthma, andrographolides (30 mg/kg, ip) inhibited the elevation of bronchoalveolar fluid (BAF) levels of TNF-alpha and GM-CSF, and almost abolished the accumulation in BAF of lymphocytes and eosinophils.89 In a similar model, andrographolide (0.1, 0.5 and 1 mg/kg, ip) dose-dependently inhibited increases in total cell count, eosinophil count and IL-4, IL-5 and IL-13 levels in BAF.90 It also attenuated IgE responses, eosinophilia and airway mucus production and hyper-responsiveness. Examination of lung tissue specimens and further in vitro investigations suggested that andrographolide might act by inhibiting the NF-kappaB pathway. An anti-inflammatory mechanism mediated by reduced NF-kappaB expression was also observed for andrographolide (2 mg/kg/day for 7 days, ip) in another study in mice, using a similar experimental model of asthma.91
Oral doses of andrographolide at 300 mg/kg demonstrated analgesic activity; at 100 and 300 mg/kg significant antipyretic effects were also observed after 3 h. In addition, this dose exhibited significant protective activity against aspirin-induced ulceration in rats.92 An aqueous extract of Andrographis (40 and 100 mg/kg, oral) and andrographolide (25, 50 and 100 mg/kg, oral), but not a 95% ethanolic extract (100 and 200 mg/kg, oral), demonstrated significant analgesic activity in mice.93 The aqueous extract and andrographolide were also active at oral doses of 100 mg/kg in reducing carrageenan-induced rat paw oedema. A similar activity profile (analgesic and antioedema) was demonstrated for subcutaneous injection of andrographolide (10, 25 and 50 mg/kg).94 Analgesia was probably mediated via non-opioid pathways, since naloxone failed to antagonise the activity of andrographolide. A methanolic extract of Andrographis (100 to 300 mg/kg, ip) slightly lowered body temperature, increased pentobarbitone sleeping time, demonstrated analgesic activity, reduced exploratory behaviour and curiosity, and exhibited some muscle-relaxing activity in mice.95 The same doses in rats also reduced exploratory behaviour in the Y-maze test.
Hypoglycaemic activity
An aqueous extract of Andrographis (10 mg/kg) was found to prevent glucose-induced hyperglycaemia in rabbits, but failed to prevent glucose absorption from the gut.96 In a screening of several traditional remedies, only Andrographis (as aqueous extract, and especially freeze-dried extract, administered at 50 mg/kg and 6.25 mg/kg body weight) significantly lowered blood glucose levels in streptozotocin-induced hyperglycaemic rats.97 In a further study Andrographis decoction was orally administered to alloxan-induced diabetic rats, with a significant reduction in blood glucose levels observed compared with controls.98
In a study comparing normal and streptozotocin-induced diabetic rats, an ethanolic extract of Andrographis not only demonstrated hypoglycaemic effects, but also reduced oxidative stress in the diabetic rats. Normal and diabetic rats were randomly divided into groups and treated orally with distilled water, metformin (500 mg/kg) or Andrographis (400 mg/kg) twice daily for 14 days. Both Andrographis and metformin significantly increased body weight and reduced fasting serum glucose in the diabetic rats, but had no such effects in the normal rats. Both treatments also significantly increased the activity of the antioxidant enzymes superoxide dismutase (SOD) and catalase in the diabetic rats, but again not in the normal rats.99 In another study, Andrographis extract (400 mg/kg, oral) decreased blood glucose and increase activities of the antioxidant enzymes SOD and catalase in streptozotocin-induced diabetic rats.100
Inhibition of the digestive enzymes alpha-glucosidase and alpha-amylase can significantly decrease the postprandial increase in blood glucose. In vitro testing demonstrated that a 20% ethanolic extract of Andrographis possessed an appreciable alpha-glucosidase inhibitory effect, but demonstrated only weak inhibition of alpha-amylase.101 Supporting this observation, a single oral dose of Andrographis extract (250, 500 or 1000 mg) dose-dependently and significantly reduced blood glucose in streptozotocin-induced diabetic rats challenged with starch and sucrose, but not after a glucose challenge.101 In contrast, andrographolide (0.5 to 1.5 mg/kg, oral) dose-dependently decreased plasma glucose in streptozotocin-induced diabetic rats, and at 1.5 mg/kg (oral) significantly attenuated plasma glucose after glucose challenge in normal rats.102
As a possible contradiction of the above results for sucrose, oral administration of Andrographis extract and andrographolide produced a dose- and time-dependent activation of brush-border membrane-bound hydrolases (lactase, maltase, sucrase) in rats, suggesting it accelerated the intestinal digestion and absorption of disaccharides.103
Other activity
An aqueous extract of Andrographis (200 mg/kg, oral) largely countered the nephrotoxic impact of gentamicin in rats, in terms of tending to normalise serum creatinine and urea, blood urea nitrogen and urine volume.104 Whether this effect was due to antioxidant activity or represented a specific renoprotective activity is not clear.
Three studies cited above attest to significant in vivo and in vitro antioxidant activity for the herb,87,93,100 as do some of the hepatoprotection studies. Oral administration of an aqueous extract of Andrographis (10, 20 and 30 mg per mouse) caused a significant elevation of catalase, SOD and glutathione-S-transferase activities in lymphoma-bearing mice, as well as exhibiting some antitumour activity.105
Other studies have demonstrated protection against toxins, in some cases linked to antioxidant activity. Andrographolide and an aqueous extract of Andrographis (250 mg/kg/day for 7 days, ip) protected against nicotine-induced neurotoxicity in rats by reducing oxidative stress.106 A similar protective activity against nicotine-induced oxidative stress was demonstrated in vitro for lymphocytes.107
A 70% ethanolic extract of Andrographis (10 mg/animal/day for 10 days, ip) was also shown to protect against cyclophosphamide toxicity in mice in two separate but similar studies conducted by the same research group.108,109 The elevation of TNF-alpha induced by the drug was lowered by Andrographis treatment. Andrographolide was also active.109
Topical application of Andrographis extract (10%) improved wound healing in rats.110 Wounds dressed with Andrographis showed markedly less scar width, higher fibroblast proliferation, more collagen, less angiogenesis and an absence of inflammatory cells.
Pharmacokinetics
An early study found that oral doses of radiolabelled andrographolide given to mice were rapidly absorbed and distributed to organs, especially gallbladder, kidney, ovary and lung. Andrographolide levels appeared to be low in spleen, heart and brain. Approximately 90% was excreted in the urine and faeces after 24 h, and 94% after 48 h. At 48 h, radiolabelled andrographolide only accounted for approximately 11% of urine and liver fractions, the remainder consisted of metabolites.111
Using isolated rat small intestine it was observed that P-glycoprotein (P-gp) was involved in the intestinal transport and absorption of andrographolide.112 One recent study using in vitro models and rats calculated that andrographolide had low absolute bioavailability (2.67%) because of its rapid biotransformation and efflux by P-gp.113 Metabolites of andrographolide found in rats included sulphates114 and an unusual sulphonic acid derivative (in urine).115
Studies in human volunteers have identified sulphate116 and glucuronide117 conjugates in urine after ingestion of andrographolide. Administration of a single 200 mg dose of andrographolide to each of 20 healthy volunteers revealed mean values of Tmax and Cmax of 1.6 h (range 1.5 to 2.0 h) and 58.6 ng/mL (range 29.3 to 81.2 ng/mL), respectively.118 The elimination half-life of andrographolide was 10.5±2.1 h. These results are consistent with a relatively low bioavailability for andrographolide.
Andrographolides might exhibit better bioavailability from Andrographis extracts. After oral administration of 1 g/kg of an extract (containing 4.52% andrographolide and 2.95% 14-deoxy-11,12-didehydroandrographolide (DIAP)) to rats, the respective Cmax and Tmax values observed were 1.42±0.09 μg/mL and 3.0±0.12 h for andrographolide and 1.31±0.05 μg/mL and 3.0±0.15 h for DIAP.119 Similarly in rabbits, 2 mL/kg of a liquid extract of Andrographis (containing 35.2 mg andrographolide and 20.7 mg DIAP) resulted in respective Cmax and Tmax values of 2.28 μg/mL and 1.0 h for andrographolide and 1.33 μg/mL and 0.75 h for DIAP.120
The amount of andrographolide was determined in the blood plasma of rats and 15 human volunteers following the oral administration of a 60% ethanolic Andrographis extract to rats and its combination with Siberian ginseng to humans (Kan Jang).121 In rats it was found that andrographolide is rapidly and almost totally (91%) absorbed after oral administration of 20 mg/kg of the Andrographis extract. Less than 10% was found in the urine, presumably because of extensive metabolism. The pharmacokinetics of andrographolide was found to be highly variable in humans after the single oral administration of 20 mg via Kan Jang, although it was reasonably rapidly absorbed (Tmax 1.37 h). The calculated steady-state plasma concentration after the normal multiple doses of the herbal combination was around 0.66 μg/mL, with a Cmax after each dose of about 1.34 μg/mL.
Clinical trials
Respiratory infections
A recent systematic review of two systematic reviews and eight clinical trials concluded that there was evidence that Andrographis was useful in the treatment of upper respiratory tract infections, but expressed concerns about publication bias and particularly the fact that most of the trials had been conducted in association with product manufacturers.122 The authors called for more independent clinical trials.
The two systematic reviews included in the above were as follows. In one, seven double blind, controlled trials (n=896) that met inclusion criteria for evaluation of efficacy were considered. All trials scored at least three (out of a maximum of five) for methodological quality on the Jadad scale. Collectively, the data suggested that Andrographis was superior to placebo in alleviating the subjective symptoms of uncomplicated upper respiratory tract infection. There was also preliminary evidence of a preventative effect. Adverse events reported following the herb administration were generally mild and infrequent.123 In the second review, 433 patients from three trials were included in the meta-analysis. Andrographis either alone or in combination with Siberian ginseng was more effective than placebo in the treatment of uncomplicated acute upper respiratory tract infection.124
In a general review of the literature for evidence of the efficacy and safety of complementary and alternative medicine for the prevention and treatment of upper respiratory tract infection in children, the authors concluded that Andrographis decreased nasal secretions (p<0.01), but not other symptoms.125
The subjects of these reviews as well as other studies follow.
Uncontrolled early Chinese clinical studies in patients with bacterial and viral respiratory infections suggested beneficial effects after oral administration of Andrographis or andrographolides, implying an immune enhancing action.5 Investigations from the Sichuan Traditional Medicine Research Institution found Andrographis exerted a beneficial effect in the treatment of infectious diseases associated with cold symptoms. The major finding was the lowered body temperature within 48 h after treatment with Andrographis. Of 84 cases of common cold, 70 achieved normal body temperature within 48 h.126
A randomised double blind study of 152 patients with pharyngotonsillitis found Andrographis (6 g/day) for 1 week to be as effective as paracetamol (acetaminophen) in relieving fever and sore throat. For both groups the difference between baseline symptoms and final evaluation was significant (p<0.0001). Lower doses of Andrographis were not as effective.127
Tablets containing a total of 1200 mg Andrographis extract (standardised to 4% andrographolides) or placebo were given to 61 patients suffering symptoms of common cold in a double blind, placebo-controlled clinical trial. After 4 days of treatment, measured symptoms were significantly reduced in the Andrographis-treated group compared to placebo: strength of disease (p=0.0001), tiredness (p=0.0001), sweating/shivering (p=0.001), sore throat (p=0.0001) and muscular ache (p=0.0001). In terms of clinical signs (rhinitis, sinus pains and headaches, lymphatic swellings), there was no significant difference between the treated and placebo groups at day 4. However, when the groups were compared over time (specifically day 0 versus day 4), there was a significant decrease in the intensity of these signs only for the Andrographis group (p<0.05). The overall reduction in the symptom score over time was also significant (p<0.01). The authors concluded that, based on their findings, Andrographis can significantly reduce the symptoms and duration of the common cold.128
In a randomised, double blind, placebo-controlled clinical trial, 107 healthy children received either Andrographis extract tablets (200 mg/day of extract, standardised to 11.2 mg andrographolide) or placebo for 3 months during the winter season. This dose corresponds to about 1 g of original herb. Analysis after the first month indicated no significant change for Andrographis treatment. However, by the third month there was a significant decrease in the incidence of colds compared with placebo (30% versus 62%; p<0.01). The relative risk of catching a cold was 2.1 times lower for the Andrographis group.129 The same research team conducted a further randomised, placebo-controlled, double blind study of an Andrographis extract at 1200 mg/day over 5 days in 158 adult patients. Visual analogue scale evaluations of the intensity of headache, tiredness, earache, sleeplessness, sore throat, nasal secretion, phlegm and frequency and intensity of cough were performed by the patients at days 0, 2 and 4 of the treatment. Using a logistic regression model of assessment, there was a significant decrease in the intensity of the symptoms of tiredness, sleeplessness, sore throat and nasal secretion in the Andrographis group at day 2, as compared with the placebo group. By day 4, a significant decrease in the intensity of all symptoms was observed for the Andrographis group. No adverse effects were observed or reported.130
In another randomised, double blind, placebo-controlled pilot study, 50 outpatients with symptoms of common cold were treated with tablets containing Andrographis extract (1020 mg/day, about 6 g of herb). The patients were advised to make their first clinic visit not later than 3 days after the occurrence of cold symptoms. After 5 days of therapy, subjective evaluation demonstrated a significantly reduced number of sick leave days (p<0.03), improved symptoms (p<0.025) and hastened recovery (p<0.05). Side effects were very few and mild.131
Recently, a randomised, double blind, placebo-controlled clinical trial observed that treatment with a standardised extract of Andrographis reduced the symptoms of uncomplicated upper respiratory tract infection.132 A total of 223 patients received either 200 mg/day of an Andrographis extract (about 2.5 g of herb, containing 60 mg of andrographolides) or a matching placebo for 5 days after experiencing the typical symptoms of a common cold. Nine self-evaluated symptoms were used to assess the efficacy of the herbal treatment: cough, expectoration, nasal discharge, headache, fever, sore throat, earache, malaise/fatigue and sleep disturbance. Both groups showed improvement in these scores from days 1 to 3. However, from days 3 to 5 most of the symptoms in the placebo group were unchanged, whereas symptom improvement continued for the Andrographis group. The difference in the overall symptom score between the two groups was significant at day 5 (p<0.05). For individual symptoms on day 5, all were significantly improved for the Andrographis group versus placebo (p<0.05), except for earache. The overall efficacy of Andrographis was a significant 2.1 times higher than placebo (p<0.05), and the herbal treatment was well tolerated. One weakness of the trial design was that patients were not treated for longer than 5 days. Hence the impact of Andrographis on shortening the duration of the common cold could not be assessed.
In two randomised, parallel-group clinical studies, a standardised extract of Andrographis (as the combination Kan Jang) was compared with amantadine in the treatment of diagnosed viral influenza infection.133 Each tablet comprised 85 mg of Andrographis extract (containing 5 mg andrographolides) and 10 mg of Siberian ginseng extract (from 120 mg root). The typical acute dose was four tablets three times daily. In the first pilot study, 71 Kan Jang-treated patients were compared with 469 patients on amantadine; in the second phase 66 patients were enrolled. Duration of sick leave and frequency of post-influenza complications were used as outcome measures and indicated that the herbal combination contributed to a quicker recovery. It reduced the risk of post-influenza complications and was also well tolerated.
A three-arm study compared the efficacy of standard treatment, Kan Jang and a preparation containing Echinacea purpurea extract in patients with uncomplicated common colds.134 Of the 130 children aged between 4 and 11 years studied over a period of 10 days, 39 patients received only standard treatment, 53 were treated with the Andrographis combination plus standard treatment and 41 were treated with Echinacea plus standard treatment. It was found that adjuvant treatment with the Andrographis combination was significantly more effective than the Echinacea preparation when started at an early stage of uncomplicated common colds. The effect was particularly pronounced in terms of the amount of nasal secretion and congestion. It also accelerated the recovery time compared to other treatments. The need for standard medication was significantly less in the Andrographis combination group compared with the others and it was well tolerated, with no adverse reactions reported.
The same Andrographis combination was also tested in a phase III randomised, double blind, placebo-controlled parallel group clinical trial in the treatment of uncomplicated upperrespiratory tract infections. After an initial pilot trial involving 46 patients over 3 to 8 days, 179 patients completed the 3-day study according to protocol. Both the total symptom score (from patients’ evaluation) and the total diagnosis score (from physicians’ evaluation) showed highly significant improvements (p<0.0006 and p=0.003, respectively), as compared with placebo. Throat signs and symptoms demonstrated the most significant improvement.135
A double blind, placebo-controlled clinical study evaluated the impact of Kan Jang treatment for 5 days in the management of acute upper respiratory symptoms (including sinusitis) in 185 patients. At the end of the treatment, significant differences compared with placebo were in evidence for total symptoms in the group as a whole (p<0.001) and in the acute and recurrent sinusitis subgroups. In terms of individual symptoms in the whole group, significant differences against placebo (p<0.001) were observed for sore throat, headache, malaise and catarrh.136
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree