(1)
Toyonaka, Japan
6.1 Introduction
Symptomatic drugs for Alzheimer’s disease (AD) target neurotransmitters.
The cholinergic nervous system is closely involved in memory and learning. The AD brain shows a conspicuous deficit of cholinergic nerve cells from the Meynert nucleus, which is combined with a reduction in acetylcholine synthesis. Therefore, the initial drug development strategy of pharmaceutical companies involved stimulating the acetylcholine system. They succeeded in developing cholinesterase (ChE) inhibitors acting as competitive inhibitors of acetylcholine degrading enzymes. This was an attempt to block the breakdown of acetylcholine, therefore increasing the amount of acetylcholine in the synaptic cleft. Drugs belonging to this category include donepezil, galantamine, and rivastigmine. In AD brains, excess excitation from glutamatergic neurons brings about neuronal death; therefore, memantine, an NMDA receptor antagonist, is also used in most clinical situations.
6.2 Characteristics of Symptomatic Drugs (Table 6.1)
Table 6.1
Symptomatic drugs for AD
Donepezil | Rivastigmine (patch) | Galantamine | Memantine | |
|---|---|---|---|---|
Cholinesterase inhibitors | NMDA receptor antagonist | |||
Mechanism | Inhibitor to acetylcholinesterase | Inhibitor to acetylcholinesterase and butyrylcholinesterase | Inhibitor to acetylcholinesterase and elevator of acetylcholine receptor sensitivity | Noncompetitive voltage-dependent antagonist on NMDA receptor |
Half-life(hr) | 70–80 | 3.3 (18 mg patch) | 5–7 | 60–80 |
Usage | 3–10 mg | 4.5–18 mg | 8–24 mg (b.i.d.) | 5–20 mg |
Metabolism | CYP 2D6 | Nonhepatic | CYP 2D6 | Nonhepatic |
CYP 3A4 | CYP 3A4 | |||
6.2.1 Donepezil
Donepezil was the first ChE inhibitor to be used clinically. As a result, we now have a good deal of evidence, safety data, and clinical usage experience. Important characteristics of donepezil are that it (1) migrates effectively to the brain; (2) works selectively on acetylcholinesterase (AChE), has little effect on peripheral butyrylcholinesterase, and induces few GI side effects; and (3) has a long half-life (70–80 hours) making it suitable for once-daily administration. In addition, it is available in a variety of dosage forms: tablets, oral rapidly disintegrating tablets, and oral jelly tablets.
Donepezil usage normally starts with one 3-mg tablet daily, followed by observation for the onset of adverse reactions. If none are evident after 2 weeks, the dose is increased and maintained at 5 mg. For patients with moderate to advanced AD, the daily dose is raised to 10 mg. Adverse reactions include upper abdominal pain, loss of appetite, and vomiting. Special caution is required when prescribing high doses.
In 2010, the US FDA approved sustained-release 23-mg donepezil, and it has been indicated for moderate to advanced AD. It causes slightly more adverse reactions than the 10-mg dose, but significantly improves patients’ cognitive symptoms. It is therefore likely that the dosage of donepezil used in clinical settings will increase to higher dosages.
Donepezil produces significant cognitive, behavioral, and global improvements in Lewy body dementia (DLB) patients. In Japan, its donepezil was expanded to DLB in 2014.
6.2.2 Galantamine
6.2.2.1 Galantamine’s Allosteric Potentiating Ligand Action
Galantamine activates the acetylcholine system by the dual action of (1) elevating synaptic cleft acetylcholine levels via its cholinesterase inhibitor action and (2) elevating nicotinic acetylcholine receptor sensitivity via the allosteric potentiating ligand (APL) action. APL is an action whereby galantamine, an allosteric modulator, binds to allosteric sites different from acetylcholine- or agonist-binding active sites and induces conformational changes at the active sites of nicotinic acetylcholine receptors. This increases the action (affinity) of agonists, such as acetylcholine (Fig. 6.1).
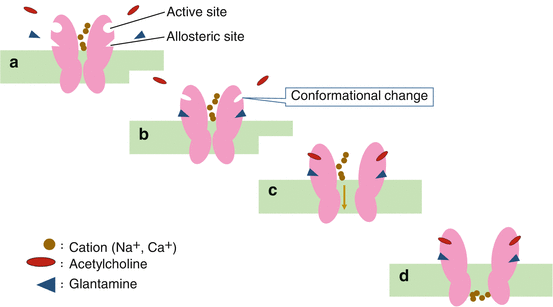

Fig. 6.1
Galantamine’s allosteric potentiating ligand action. (a) Resting state of nicotinic acetylcholine receptor. (b) Glantamine binds to allosteric sites and induces conformational changes at active sites. (c) Acetylcholine favorably binds to active sites. (d) The channels opens to allow cations to pass through the membrane
Besides increasing signal transmission sensitivity, a drug with APL actions may have significant effects like (1) saturation of the active site, preventing overactivation (“ceiling effect”), (2) blockage of signal transmission unless a ligand already exists, (3) enhanced receptor selectivity for subtypes, and, lastly, (4) it is not liable to cause receptor desensitization or downregulation. Therefore, the drug could possibly enhance signal transmission safety.
6.2.2.2 Galantamine Activation of Nicotinic Acetylcholine Receptors
Because of galantamine’s APL effects on nicotinic acetylcholine receptors, activation of the nicotinic acetylcholine receptor itself can be expected. When activating nicotinic acetylcholine receptors present in the presynaptic membrane, galantamine promotes the release of neurotransmitters such as norepinephrine, serotonin, glutamate, and γ-aminobutyric acid (GABA). Thus, it influences a person’s psychiatric condition and is expected to have a parallel effect on the behavioral and psychological symptoms of dementia (BPSD).
Activation of nicotinic acetylcholine receptors may appear to bring about neuroprotective effects. In one study, a 13-week administration of galantamine resulted in significantly lower levels of tau protein in the cerebrospinal fluid (CSF), an indicator of nerve damage, than treatment with donepezil (Nordberg et al. 2009).
Activation of nicotinic acetylcholine receptors also induces a rise in vasomotor reactivity; therefore, it is hoped to be applied to treat vascular dementia (Bär et al. 2007).
6.2.2.3 Galantamine Usage
Galantamine usage begins with a daily dose of 8 mg (b.i.d.) for mild to moderate AD. The dose is increased to 16 mg per day (b.i.d.) after 4 weeks. This may be further raised to 24 mg per day (12 mg b.i.d.), if symptoms require. Dosage increases should be done only after having administered the pre-change dose continuously for >4 weeks. This drug is also anticipated to have minimal adverse effects on peripheral butyrylcholinesterase and induces only minor gastrointestinal (GI), such as nausea and anorexia, and other adverse reactions.
6.2.3 Rivastigmine
6.2.3.1 Rivastigmine’s Actions: Inhibition of AChE and Butyrylcholinesterase
Rivastigmine has effects on both AChE and butyrylcholinesterase. Most ChEs in the normal brain are AChE, with butyrylcholinesterase making up approximately 10 %. In the hippocampus, however, butyrylcholinesterase is present at relatively high levels. Although AChE is expressed in neurons, as AD advances its activity increasingly declines due to a deficit of neurons. On the other hand, because glial cells proliferate, butyrylcholinesterase activity shows a relative rise (Table 6.2) (Perry et al. 1978). The fact that rivastigmine has inhibitory actions on both enzymes works to its advantage.
Age (years) | Postmortem delay (h) | Temporal cortex (μmole/h/mg protein) | Hippocampus (μ mole/h/mg protein) | |||
|---|---|---|---|---|---|---|
AChE | BuChE | AChE | BuChE | |||
Normal | 73 ± 4 | 30 ± 7 | 2.49 ± 0.24 | 0.233 ± 0.024 | 3.46 ± 0.40 | 0.285 ± 0.049 |
Alzheimer | 72 ± 4 | 36 ± 9 | ***1.67 ± 0.26 | *0.327 ± 0.035 | *2.15 ± 0.41 | **0.471 ± 0.040 |
6.2.3.2 Rivastigmine Transdermal Patch
The butyrylcholinesterase-inhibitory activity of rivastigmine is disadvantageous with regard to GI reactions compared with other ChE inhibitors. This is why capsule agents through the GI are refrained from using. A transdermal patch has recently been developed to lengthen the time for the drug to reach maximum blood concentration, since drug transfer to the bloodstream is slower from a patch compared to oral administration. The incidence of GI adverse reactions has therefore been reduced.
Rivastigmine usage begins with a daily dose of 4.5 mg. It is then increased by 4.5 mg every 4 week. For well-tolerated patients, it begins with 9.0 mg and 4 weeks later can be increased by 18 mg. An 18 mg patch can be applied to normal healthy skin on the back, upper arm, or chest daily as a maintenance dose and replaced every 24 hours. The dose may be increased (not to exceed 18 mg) or decreased in response to the patient’s symptoms.
6.2.4 Memantine
6.2.4.1 Abnormalities of Glutamate NMDA Receptors in the AD Brain
In the AD brain, the concentration of glutamate rises continuously due to the actions of β-amyloid and other substances. They constantly act on NMDA receptors, causing excitotoxicity and synaptic noise that impairs calcium homeostasis. Glutamate excitotoxicity is one of the hypothesized mechanisms for neurodegeneration in AD.
With NMDA receptors, moreover, high concentrations of glutamate are released during long-term potentiation (LTP), causing temporary calcium influx and producing a signal. In the AD brain, however, the concentration of glutamate is chronically high, with synaptic noise occurring all the time. This LTP signal-masking noise is thought to lead to memory disturbances (Fig. 6.2).
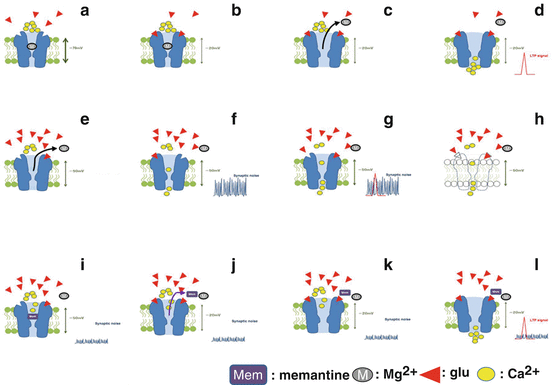

Fig. 6.2
Memantine is a noncompetitive voltage-dependent antagonist. (a) The ion channel is blocked by a magnesium (Mg2+) under resting conditions. (b) When the NMDA receptor is activated by glutamate (glu), the postsynaptic neuron is depolarized (−70 mV → −20 mV). (c) Under the depolarization, magnesium leaves the ion channel. (d) The removal of magnesium causes a calcium influx, which is important for a long-term potentiation (LTP) signal. (e) In pathological conditions, such as in Alzheimer’s disease, an increase of glutamate occurs in the synaptic craft resulting in continuous activation of NMDA receptors by lower concentrations of glutamate. The incomplete activation of the NMDA receptor depolarizes the postsynaptic neuron (−70 mV → −50 mV) and also releases magnesium from the ion channel. (f) The incomplete but continuous stimulation of NMDA receptor leads to an uncoordinated calcium influx, which enhances postsynaptic noise. (g) When a relevant LTP signal is presented, it is buried in the noise. This results in a deficit in cognitive function. (h) The stimulation of NMDA receptor with continuous calcium influx ultimately leads to damage of neurons. (i) By less pronounced voltage dependence of memantine (Mem) than magnesium, it can block the calcium influx under incomplete depolarization (−50 mV) of the postsynaptic neuron. Therefore memantine exerts a neuroprotective effect as well as suppresses synaptic noise. (j) However, under the strong depolarization causing LTP, memantine can dissociate from the ion channel due to its voltage dependency. (k) By a dissociation of memantine, the ion channel is ready for a surge of calcium influx. (l) The calcium influx generates LTP signal
6.2.4.2 Memantine Is a Noncompetitive Voltage-Dependent Antagonist
Memantine is a noncompetitive (open-channel) NMDA receptor antagonist with low to moderate affinity. Memantine’s inhibitory action on NMDA receptor activity is dependent on membrane potential. During physiological neuronal excitation, glutamate is briefly released at high concentrations, elevating the postsynaptic membrane potential. Under these conditions, memantine easily dissociates from the NMDA receptor and does not impede physiological LTP. On the other hand, due to the continuous release of glutamate at relatively low concentrations under pathologic conditions, the membrane potential remains low in AD. Memantine is therefore believed to exert its neuroprotective effects as well as suppresses synaptic noise by inhibiting NMDA receptors. Memantine also allows the relevant physiological LTP signal to be detected. Both neuroprotection and symptomatic restoration of synaptic plasticity by memantine are provided by the same mechanism (Fig. 6.2).
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


