41 HIV infection
Pathogenesis
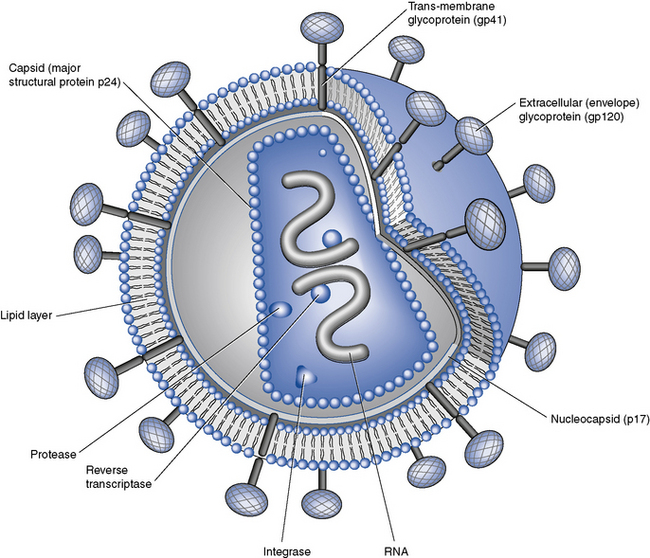
HIV, in common with other retroviruses, possesses the enzyme reverse transcriptase and consists of a lipid bilayer membrane surrounding the capsid (Fig. 41.1). Its surface glycoprotein molecule (gp120) has a strong affinity for the CD4 receptor protein found predominantly on the T-helper/inducer lymphocytes. Monocytes and macrophages may also possess CD4 receptors in low densities and can therefore also be infected. The process of HIV entry is more complex than originally thought, and in addition to CD4 attachment, subsequent binding to co-receptors such as CCR-5 or CXCR-4 and membrane fusion also occur (Fig. 41.2).
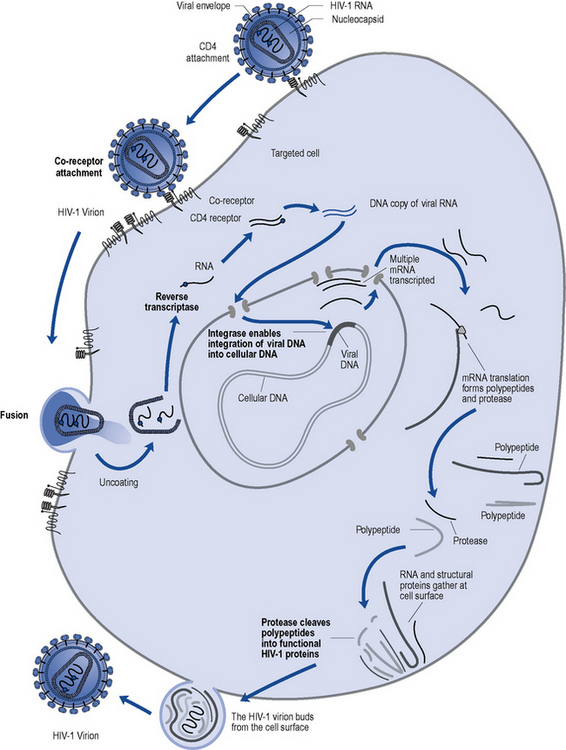
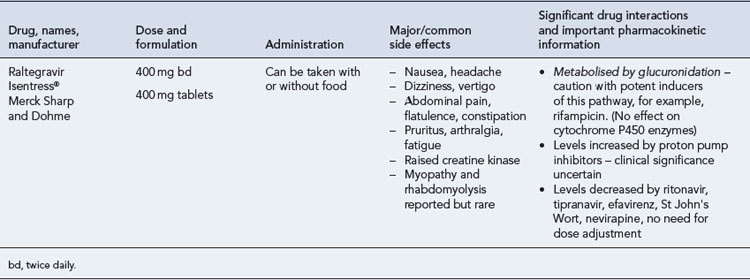
Fig. 41.2 Lifecycle of HIV and the sites of action of currently available antiretroviral agents (in bold).
Clinical manifestations
The sequelae of untreated HIV infection can be broadly considered in five categories:
Opportunistic infections generally fall into two categories:
Investigations and monitoring
CD4 count
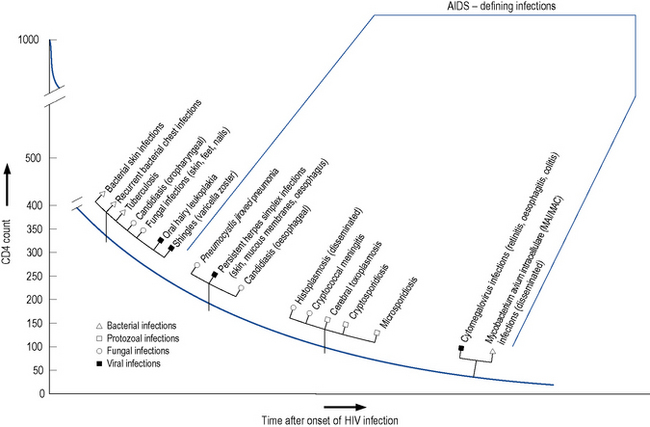
The level of immunosuppression is most easily estimated by monitoring a patient’s CD4 count. This measures the number of CD4-positive T-lymphocytes in a sample of peripheral blood. The normal range can vary between 500 and 1500 cells/mm3. As HIV disease progresses, the number of cells falls. Particular complications of HIV infection usually begin to occur at similar CD4 counts (Fig. 41.3) which can assist in differential diagnoses and enable the use of prophylactic therapies. For example, patients with a CD4 count of less than 200 cells/mm3 should always be offered prophylaxis against P. jiroveci pneumonia. Similarly, both patient and clinician are likely to use the CD4 count as the major indicator of when to consider starting antiretroviral therapy.
Viral load
The measurement of plasma HIV RNA (viral load) estimates the amount of circulating virus in the blood. This has been proven to correlate with prognosis, with a high viral load predicting faster disease progression (Mellors et al., 1997). Conversely, a reduction in viral load after commencement of antiviral therapy is associated with clinical benefit. This measure, in combination with the CD4 count, allows patients and clinicians to make informed decisions regarding when to start and when to change antiviral therapies, enabling the more effective use of such agents. There are on-line calculators utilising viral load and CD4 count to model risk of disease progression or death based on large cohort studies.
Drug treatment
The goals of therapy in HIV-positive individuals are to:
Antiretroviral therapy
Many organisations, such as the British HIV Association (BHIVA), the European AIDS Clinical Society (EACS) and the International AIDS Society (IAS), produce regularly updated guidelines on the use of antiretroviral therapy, for example, Gazzard et al. (2008). These guidelines include the most up-to-date considerations of:
Most studies evaluating triple combinations of antiretrovirals have been designed with so-called surrogate marker endpoints, measuring the effect on laboratory parameters such as CD4 count and HIV viral load. These trials are generally smaller and shorter in duration than clinical endpoint studies that are powered to measure the impact on survival and disease progression. The first large clinical endpoint trial that demonstrated the superiority of a triple combination over dual therapy was undertaken by Hammer et al. (1997). Following the results of this trial, the standard approach, where treatment is indicated, has been to use a combination of at least three agents. The reduction in morbidity and mortality associated with HAART has been confirmed in routine clinical practice, as well as in other trials (e.g. Palella et al., 1998; Smit et al., 2006). Subsequent clinical trials have largely been for licensing purposes and/or have served to refine therapeutic choices rather than to change the paradigm of treatment. The concept of intermittent rather than continuous therapy was evaluated in the SMART study but shown to be linked with an increased risk of co-morbidities not previously thought to be associated with HIV (such as cardiovascular disease, hepatic and renal failure) as well as HIV disease progression (El-Sadr et al., 2006). The use of protease inhibitor (PI) ‘monotherapy’ compared to conventional triple therapy has been evaluated in a number of small studies, for example, Arribas et al. (2009), and is being investigated in longer-term strategic studies. A large international study of early versus deferred treatment, to attempt to address the question of when to initiate treatment, is ongoing.
Choosing and monitoring therapy
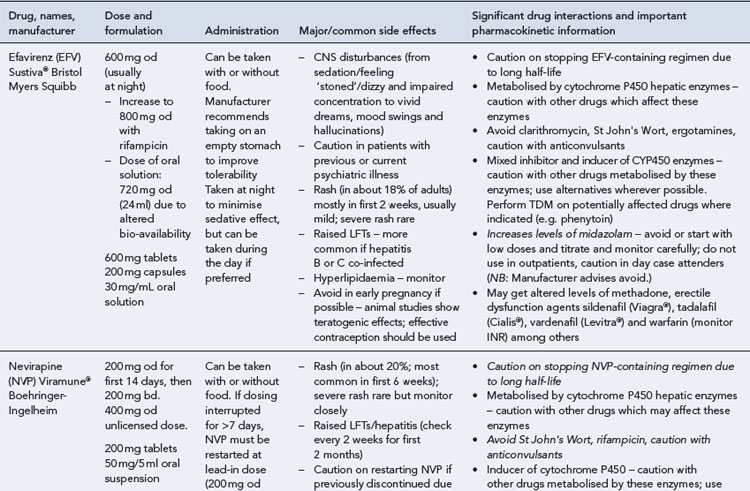
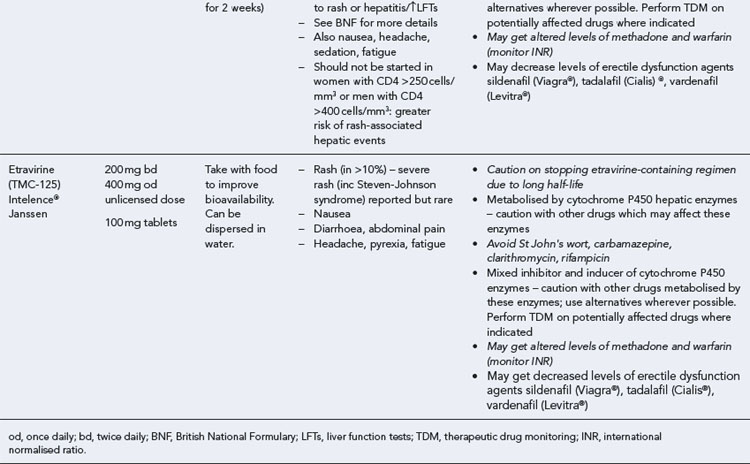
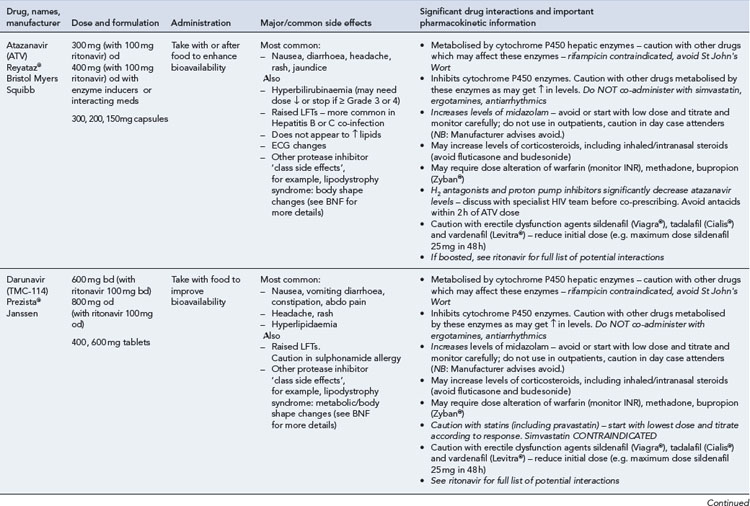
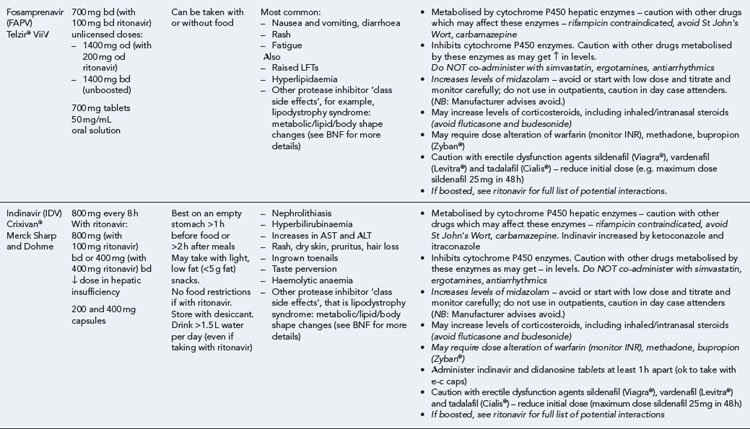
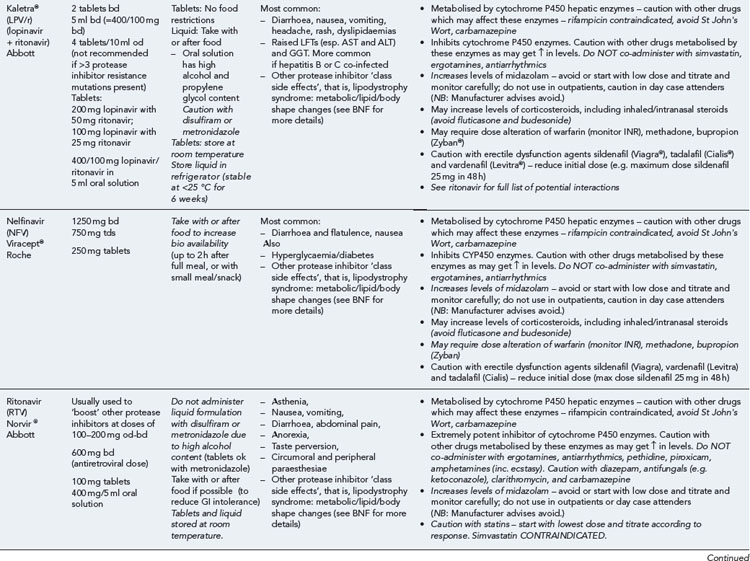
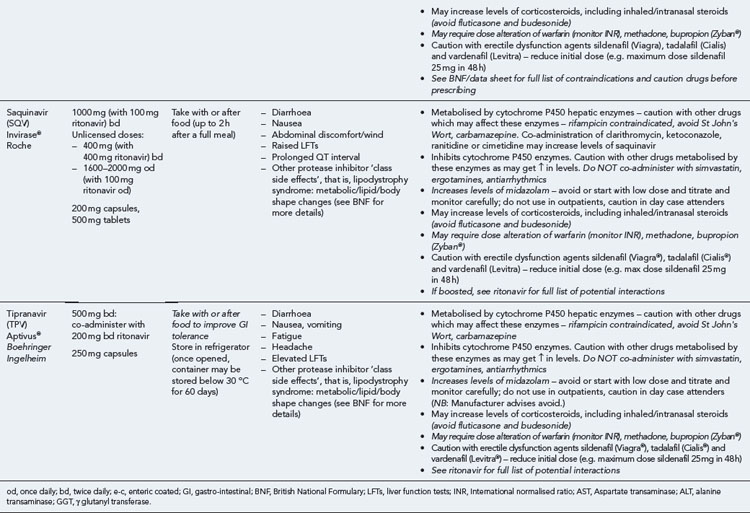
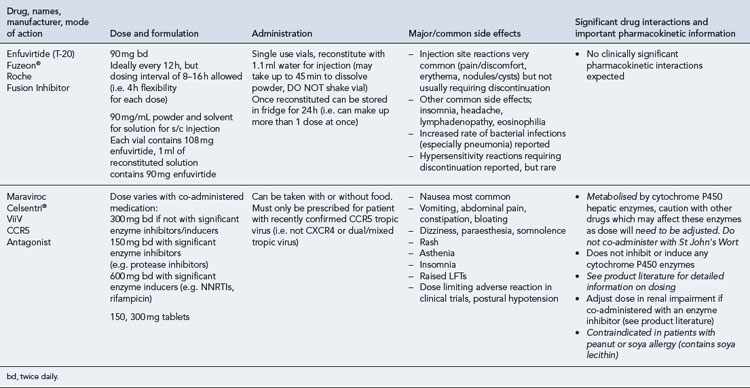
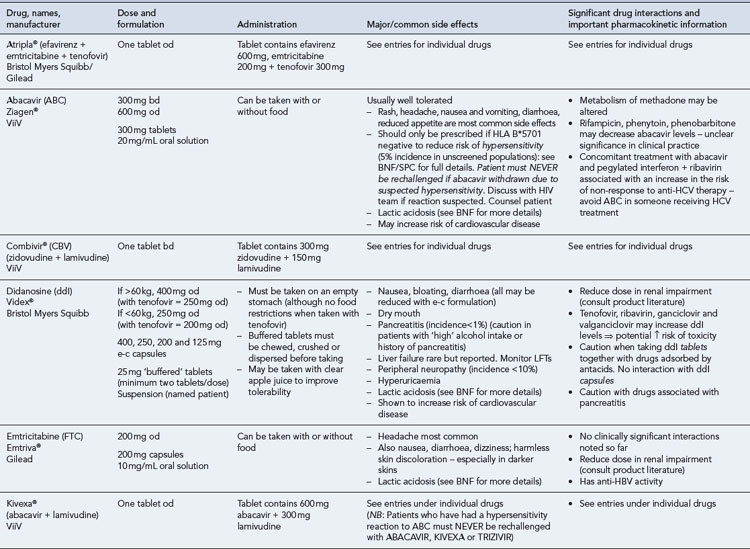
Many of the antiretrovirals, particularly the PIs and NNRTIs, exhibit a wide range of interactions, especially with other drugs that are metabolised by the cytochrome P450 enzyme system, including prescribed, ‘over-the-counter’, herbal and recreational drugs. HAART failure (detectable viral load and drug resistance) has been documented following co-administration of hepatic enzyme inducers, including non-prescribed agents such as St John’s Wort. Conversely, serious and even fatal toxicities due to enzyme inhibition by the PIs continue to be reported. These include Cushing’s syndrome following concomitant use of fluticasone or budesonide inhaler or nasal spray with a PI. This highlights the necessity of taking a comprehensive drug history prior to starting or switching HAART and ensuring patients and prescribers are aware of the need to check the interaction potential of new medicines. General prescribing guidelines for antiretrovirals are presented in Box 41.1 whilst details of common side effects and interactions of the currently available agents are summarised in Tables 41.1 to 41.5.
Box 41.1 General prescribing and monitoring information for antiretroviral agents
Table 41.1 General prescribing points for nucleoside/nucleotide analogue reverse transcriptase inhibitors (NRTIs)
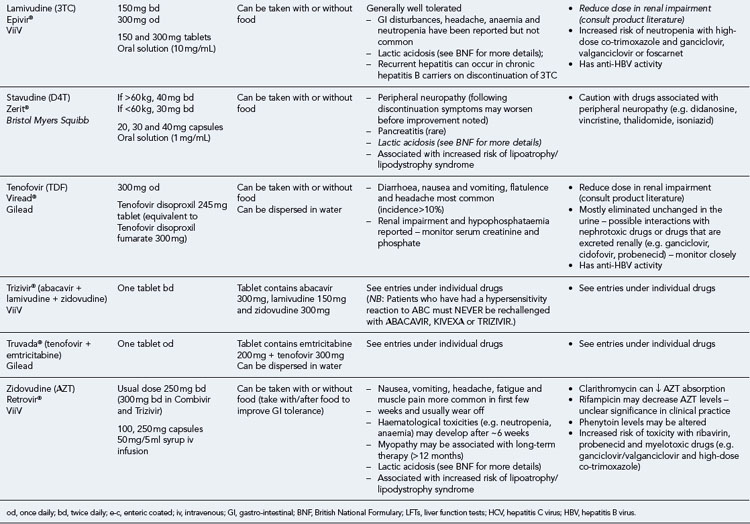
The routine use of therapeutic drug monitoring is not recommended but blood levels of PIs and NNRTIs should be measured in selected patients, for example, during pregnancy, where there is liver impairment and where there are concerns regarding potentially interacting drugs (Gazzard et al., 2008).
HIV mutates readily and resistance to some antiretrovirals, particularly reverse transcriptase inhibitors and integrase inhibitors, develops rapidly in the face of suboptimal treatment, for example, monotherapy or subtherapeutic blood levels. A high level of adherence to treatment is crucial to the sustained, successful outcome of antiretroviral regimens and has been the subject of much research. For example, in one study of people taking their first regimen containing nelfinavir, it was found that at least 95% adherence was required to achieve a sustained response in the majority (78%) of patients. The chances of treatment success declined as the level of adherence dropped, such that 80% of patients whose adherence was below 80% experienced virological failure. Virological success was also found to correlate with a better clinical outcome in terms of fewer hospitalisations, opportunistic infections and deaths (Paterson et al., 2000). Such clinical trial data have also been supported by clinical experience in the UK and elsewhere, although it has yet to be established if the level of adherence required is the same for all regimens and every patient. In view of this, patients should be advised to take HAART as close as possible to the same time every day and certainly within 1 hour of the agreed time each day. If they forget a dose, it should be taken as soon as they remember and then return to the original schedule.
There has been significant progress over recent years in reducing some of the physical burden of therapy, through the development of combination tablets and the use of strategies such as ritonavir boosting to reduce dietary restrictions and dosing frequency. Adherence aids such as pill boxes, medication record cards and alarms (e.g. on mobile phone) can also help to support adherence. However, practical issues are not the only barriers to adherence and the individual’s health beliefs and motivation, particularly around HIV and antiretroviral therapy, should also be addressed before treatment is commenced, as these are likely to have a significant impact on outcome (Horne et al., 2004). Although there is little evidence to demonstrate what the optimal interventions to improve adherence are, multidisciplinary and multiagency approaches appear to be most useful (Poppa et al., 2003).
Treatment interruptions
For many reasons, including toxicity, cost and adherence, patients and clinicians have been interested in considering ‘drug holidays’ or treatment interruptions. However, this strategy is no longer recommended in routine practice (El-Sadr et al., 2006). It is now recognised that there are dangers associated with this approach because of CD4 decline, disease progression, mortality related to co-morbidities, for example, cardiovascular disease, and viral load rebound associated with increased transmission risk and a seroconversion-like syndrome. Further, as different anti-HIV medications have different half-lives, there may be a risk of functional monotherapy, particularly with NNRTIs, and the development of resistance if combinations are stopped abruptly in an unplanned fashion.
Post-exposure prophylaxis
Post-exposure prophylaxis (PEP) involves the use of antiretroviral drugs to prevent infection with HIV after possible exposure, which may be recommended after occupational injuries (DH, 2008) or sexual exposure (Fisher et al., 2006). Whilst PEP is a largely unproven and unlicensed indication for the drugs used, it is supported by animal model data and case–control studies. Where recommended in guidelines, PEP is usually commenced as a 3–5-day starter regimen of two NRTIs and a boosted PI, followed by an ongoing course for a total of 4-week post-exposure. It is believed this will reduce the likelihood of infection by at least 80%, although toxicity issues are not insignificant. Therefore, the decision to prescribe or take PEP must reflect a careful risk/benefit evaluation. Studies of pre-exposure prophylaxis (PREP), using one or two antiretrovirals (orally or topically) before potential exposure to HIV, have so far yielded mixed results, but may offer additional options to reduce transmission.
Nucleoside and nucleotide analogue reverse transcriptase inhibitors
There is also one formulation combining two NRTIs with an NNRTI:
Other triple and quadruple mixed class co-formulations are in development.