and Alena Skalova2
(1)
Departamento de Ciências Biomédicas e Medicina, Universidade do Algarve, Faro, Portugal
(2)
Department of Pathology, Medical Faculty Charles University, Plzen, Czech Republic
1.1 Salivary Glands
The salivary glands consist of the paired parotid, submandibular and sublingual glands, and numerous minor salivary glands diffusively dispersed in the entire upper aerodigestive tract. The anlage for the parotid and submandibular glands appears in the sixth/seventh week; the sublingual gland appears in the ninth. The parotid gland is of ectodermal origin whilst the submandibular and sublingual glands more likely are of endodermal derivation. The adult parotid gland is the largest and weighs between approximately 25 g, the submandibular 7–8 g, whilst the principal sublingual only approximately 3 g. The parotid gland is located below the external acoustic meatus, between the mandible and the sternocleidomastoid. The gland is divided into two portions by the facial nerve and the outer, flattened superficial lobe being the main portion. The deep lobe is irregularly wedge-shaped in anatomical relationship with the parapharyngeal space. The gland projects forwards on the surface of the masseter where a small part of it that is more or less detached lies between the zygomatic arch above and the parotid duct below. This detached part is called the accessory part of the parotid gland. The secretion of the accessory parotid is emptied by an independent duct reaching the main parotid duct, Stensen’s duct, in the masseter region. Stensen’s duct, located in the anterior portion of the gland, is 5–7 cm long and follows a twisted course, crossing both the masseter and buccinator muscles before opening into the oral vestibule. The wall of Stensen’s duct is thick and consists of an external coat with smooth muscle fibres. The canal is about 3 mm in diameter. The glandular acini are purely serous and the parenchyma is divided into lobes and lobules by numerous septa. Also the submandibular gland consists of a larger superficial part and a smaller deep part which are continuous with each other around the mylohyoid. The gland is finely encapsulated and lies inside the submandibular triangle. Like the parotid gland, it is organised in lobules connected to a main duct called Wharton’s duct. Wharton’s duct is smaller than Stensen’s duct and measures approximately 5 cm in length and 2–3 mm in diameter. The duct runs between the mylohyoideus, the hyoglossus, and genioglossus muscles and opens in a small papilla (caruncula sublingualis) situated on each side of the frenulum linguae in the floor of the mouth. The sublingual gland lies beneath the mucous membrane of the floor of the mouth, in contact with the sublingual fossa of the mandible, and is surrounded by loose connective tissue. Like the submandibular gland it is a mixed seromucinous gland but is predominantly mucous in contrast to the submandibular gland that is predominantly serous. It has the shape of an almond and its secretion is drained from the anterior part of the gland through a main duct called Bartholin’s duct which opens into the Wharton’s duct of the submandibular gland. The sublingual gland also has a number of smaller excretory ducts, Rivini’s ducts, that most often open separately into the floor of the mouth [45, 63]. The majority of the minor salivary glands are mixed seromucinous glands with the exception of Von Ebner’s gland in the tongue being purely serous and some palatal and lingual glands that are purely mucous (Table 1.1).
Table 1.1
A guide to the anatomical site and histology of salivary glands
Gland | Location | Duct opening | Histology |
|---|---|---|---|
Parotid | Styloid process-mandible | Vestibule | Pure serous |
Mastoid process | Stensen’s duct | ||
Submandibular | Submandibular triangle | Floor of the mouth (side of frenulum) | Mixed, predominantly serous |
Wharton’s duct | |||
Sublingual | Floor of the mouth | Plica sublingualis | Mixed, predominantly mucous |
Bartholin’s duct | |||
Wharton’s duct | |||
Rivinus’ duct | |||
Tongue | Tongue, circumvallate papillae | Circumvallate groove | Pure serous |
Von Ebner’s gland | |||
Weber’s gland | Base of tongue | Tongue | Pure mucous |
Posterior lingual | Tongue | Tongue | Mixed |
Anterior lingual | Tongue | Tongue | Mixed |
Blandin’s or Nuhn’s gland | |||
Buccal | Cheek | Vestibule | Mixed, predominantly mucous |
Labial | Upper and lower lips | Vestibule | Mixed, predominantly mucous |
Glossopalatine | Anterior faucial pillar | Pure mucous | |
Glossopalatine fold | Pure mucous | ||
Palatal | Hard palate | Palate | Pure mucous |
Soft palate | Pure mucous | ||
Uvula | Mixed, predominantly mucous | ||
Nasal and paranasal | Nose and paranasal sinuses | Mucosal membrane | Mixed |
Respiratory tract | Nasopharynx, pharynx, larynx and trachea | Mucosal membrane | Mixed |
The juxtaoral organ of Chievitz, described by the Danish anatomist Johan Henrik Chievitz in 1855, has been widely mistaken for the parotid anlage. It develops from the buccal sulcus and may have a neurosecretory function. It often degenerates soon after birth but may persist as a small structure in the buccotemporal space. It consists of small nests of squamous epithelium delineated by basement membrane. Larger nests may have clear cell differentiation and occasionally gland-like lumina may be present. These usually nonkeratinising epithelial nests are surrounded by Schwann cells and nerves and can be misinterpreted as perineural invasion by carcinoma [46, 62].
1.2 Secretory Units and Ducts
The acinar units are purely serous, purely mucous or mixed. Although the serous acini consist of rather large cells, the acini are smaller than those of mucous acini. The slightly triangular serous cells have their base directed outwards and form pear-shaped groups of cells surrounded by a distinct basement membrane. The cells have basal nuclei and a dense cytoplasm filled with basophilic PAS-positive zymogen granules. The number of granules correlates with the phase of the secretory cycle. The zymogen granules are composed mainly of amylase (ptyalin) which splits starch into water-soluble carbohydrates. The lacrimal gland cells, on the other hand, do not contain zymogen but eosinophilic lysozyme-producing granules. Besides amylase, a large number of different enzymes have been demonstrated in the serous cells, e.g. glucosidase, esterases, lactoferrin, lysozyme, etc. The essence of the saliva is produced in the acini and then mixed with water and electrolytes in the striated duct. The parotid secretion becomes a watery liquid that contains mainly proteins, ptyalin and salts but no mucous. The acinar cell in a mucinous acinus is also a relatively large cell that does not typically have the triangular shape as the serous cell. It contains vacuoles, and its clear cytoplasm is rich in both acid and neutral sialomucins staining positively for alcian blue and mucicarmine and for PAS, respectively. Mixed acini are typically found in the submandibular gland but also in most minor salivary glands (Table 1.1). They are characterised by the concentration of mucous cells near the intercalated duct and surrounded by a crescent-shaped formation of serous cells, the so-called serous demilunes or crescents of Giannuzzi. In contrast to the serous cell, the plasma membrane of the mucous cell is ultrastructurally seen to be devoid of microvilli. The acini are surrounded by a more or less developed basement membrane inside which there are scattered myoepithelial cells partly forming a network (Fig. 1.1).
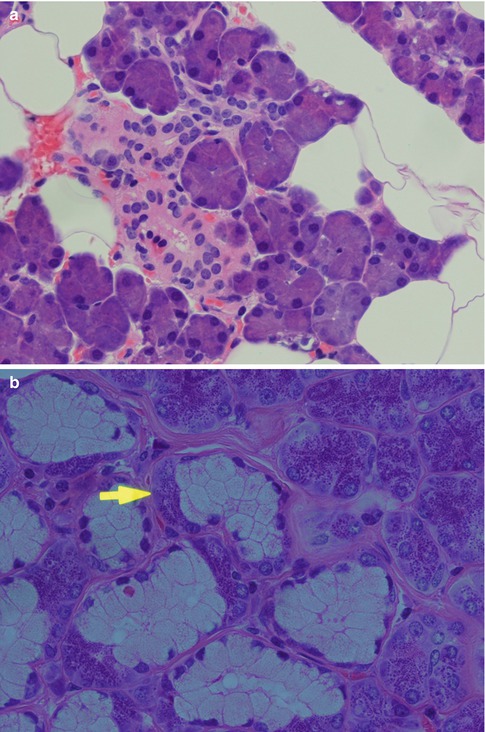
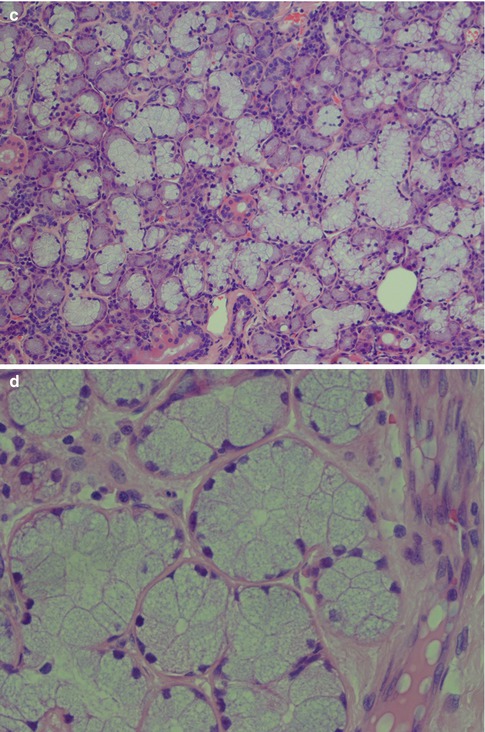


Fig. 1.1
Acinar units. (a) Pear-shaped parotid pure serous acini consisting of triangular granular cells with their base directed outwards and basal nuclei. Central lumen hardly visible. (b) Submandibular mixed seromucous acini with crescents of Giannuzzi (arrow). (c) Sublingual gland with considerably more mucous acini than in the submandibular gland. (d) Pure mucous palatal gland with acini composed of large cells of varying sizes, some of triangular shape. Nuclei situated towards the base and central lumen visible in several acini
The salivary duct system transports the saliva from the acini to the opening in the mucous membrane. The part nearest to the acinus is called the intercalated duct which connects with the striated duct. Both these ducts are intralobular and known as secretory ducts due to their metabolic activity. The striated ducts are connected with interlobular ducts located in the septal connective tissue, called excretory ducts. The relative size and length of the different ducts vary, and, for example, the parotid intercalated ducts are longer than those seen in the sublingual and submandibular gland, whilst the striated ducts in the submandibular gland are longer than their parotid counterparts. The excretory ducts’ main function is to transport saliva but their cells are hypothetically undifferentiated pluripotential cells. They may therefore be implicated in neoplastic alterations (primarily papilloma, mucoepidermoid carcinoma, salivary ductal carcinoma and squamous cell carcinoma). The transition from acinus to intercalated duct is gradual, and the complex formed by the acinus, the intercalated duct and associated myoepithelial cells, is often referred to as the terminal duct. The intercalated duct lies in contact with the acinus and consists a single layer of cuboidal cells with relatively large nuclei and few cytoplasmic organelles but with a strong cytoplasmic activity of lactoferrin and lysozyme [37]. An irregular layer of myoepithelial cells is surrounding the cuboidal epithelium. The rather long parotid intercalated ducts are relatively easily seen in routine sections but being short in the submandibular and hardly visible in the sublingual gland. It is most often seen when sectioned longitudinally. The intercalated duct is regarded as an important part of the salivary gland tissue with regard to the development of many salivary neoplasms [16]. The striated duct consists of one layer of columnar cells with centrally located nuclei. They have characteristic parallel striations on the basal side, caused by cell membrane invaginations and mitochondria. The cytoplasm of the striated ductal cells is frequently eosinophilic due to the high content of mitochondria. The large collecting interlobular ducts, the excretory ducts, are located in the septal connective tissue. They consist of pseudostratified columnar epithelium and have sparse interspersed goblet cells. Near the opening the excretory duct becomes squamous and stratified (Fig. 1.2). The acini and intercalated ducts have surrounding myoepithelial cells, whilst the striated and excretory ducts have basal cells. There are profound morphological and functional differences between myoepithelial and basal cells [8, 32, 33, 52].
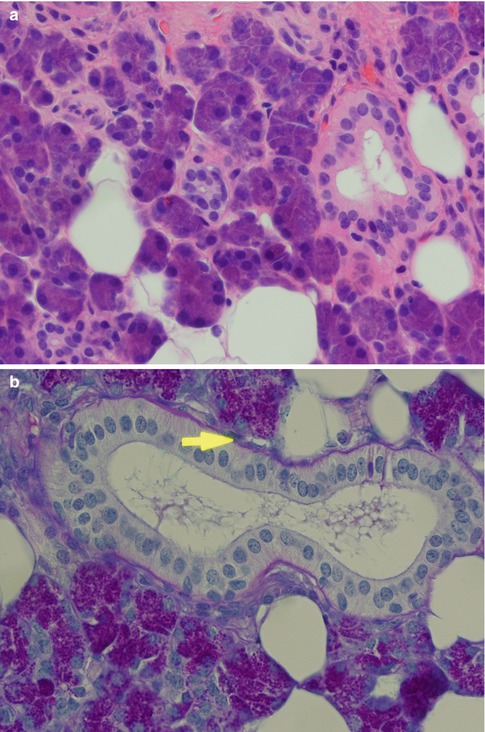
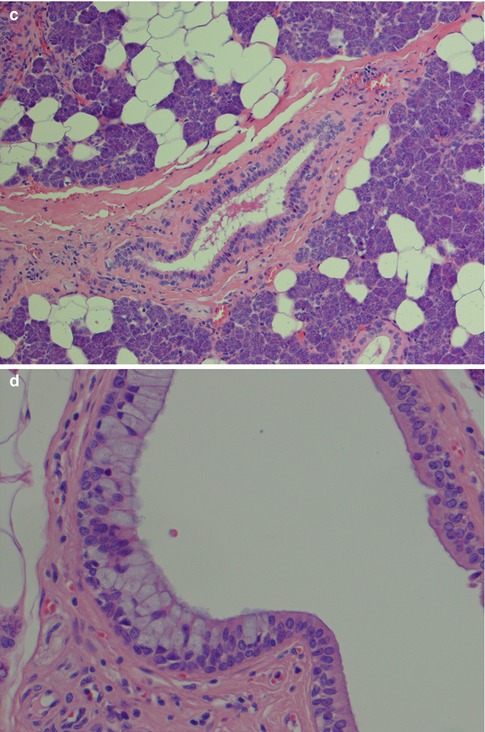


Fig. 1.2
Salivary ducts. (a) The intercalated duct has relatively large nuclei (centre), and a striated duct (right). (b) Striated duct in parotid gland. Columnar cells with central nuclei and basal-striated appearance. The duct is surrounded by thin basal membrane and irregularly placed myoepithelial cells (arrow) (PAS). (c) Excretory duct in the septal connective tissue. (d) Excretory duct with numerous goblet cells
The myoepithelial cells lie between the epithelial cells and the basal lamina of acini, intercalated duct, and probably also exist in the union of the intercalated and striated ducts [45, 52]. The cells are rather flat and have cytoplasmic processes extending over the epithelial surface in a kind of network that sometimes makes them difficult to discern in ordinary H&E sections. As for today, there is no single immunohistochemical marker that is specific for myoepithelial cells. The myoepithelial cell is of ectodermal origin but can be regarded as a cell modified by a mesenchymal potential with a fine structure similar to a smooth muscle. It is generally accepted that the myoepithelial cells are contractile and capable of glycogen storage. The myoepithelial cells may be attached to the neighbouring epithelial cell by desmosomes, at the same time merging with the connective tissue. The myoepithelial cell contains fibrils similar to myofibrils composed of microfilaments measuring up to 7 nm and consisting of actin, tropomyosin and myosin. It also contains an intermediate filament of 10–12 nm width, consisting of both pre-keratin and vimentin. Cytokeratin 14 appears to be the most consistently expressed cytokeratin. The presence of vimentin is not constant and is in fact rather often absent in normal myoepithelial cells but appears in several salivary neoplasms with myoepithelial participation. Desmin is not present. The normal myoepithelial cell is a rather small, flat, elongated cell sometimes with a dendritic appearance. As it is considerably flattened and spread out over the acini and ducts, the myoepithelial cell is often difficult to appreciate in routinely prepared salivary gland tissue. In neoplasms, a variety of cell morphologies of the myoepithelial cell have been described. These cell types include the stellate or myxoid, spindle-shaped or myoid, epithelioid, plasmacytoid or hyaline, and clear cell type (see, e.g. Chaps. 3 and 4). The spindle cells are individually defined spindle-shaped cells with abundant eosinophilic cytoplasm. The epithelioid (or epithelial) type of modified myoepithelial cells are polygonal-shaped cell with prominent cytoplasm. The plasmacytoid or hyaline cell has a distinctive appearance being an ovoid to polygonal cell with an eccentrically placed nucleus and eosinophilic hyaline cytoplasm resembling a plasma cell. They are ultrastructurally characterised by focal desmosomes, basal lamina, and abundant intermediate cytoplasmic filaments. Dense bodies typical of smooth muscle cells and actin-sized filaments are absent [25, 39]. The clear cells have a faintly or nonstaining, clear cytoplasm. The clear cytoplasmic appearance results from extensive accumulations of glycogen and widened intercellular spaces [15]. The immunoprofile of the myoepithelial cell differs somewhat according to its morphological subtype. The traditional perception of the immunoreactivity of normal myoepithelial cells is that they are positive for actin-myosin, cytokeratin and S-100. These markers and also vimentin have been found to be expressed differently in the different morphological subtypes occurring in neoplasms. In the stellate cell type, the order of frequency of expression is S-100, vimentin and actin-myosin; the spindle-shaped myoepithelial cells vimentin, actin-myosin and cytokeratin; the clear cell type cytokeratin, actin-myosin and S-100; and the plasmacytoid type cytokeratin, vimentin and S-100 [45]. Since the mid-1990s, several other markers have been used both in the breast and salivary gland pathology and in particular smooth muscle-specific proteins, such as smooth muscle actin, smooth muscle myosin heavy chain, calponin and h-caldesmone. CD10, p63, GFAP and maspin are other useful markers of the myoepithelial cell. A rather recently described myoepithelial marker is nestin which stains a neuroepithelial stem cell protein (nestin) apparently present in the cytoplasm of myoepithelial cells [36]. Most of the above markers are relatively sensitive, but none is specific for the myoepithelial cell, and both the specificity and sensitivity of these markers vary widely. Therefore, a smaller panel of antibodies is needed for the identification of myoepithelial cell participation in salivary neoplasms. The composition of an antibody panel will hence depend on which morphological type(s) of myoepithelial cell is present. S-100, vimentin and cytokeratins (particularly keratins 5,7,14 and 17) are still useful [6, 24, 26, 28, 35, 47, 48]. However, the cytokeratin staining of myoepithelial cells in normal salivary gland tissue is often very difficult to interpret due to synchronous positive staining of the ducts and in some instances positive staining also of acini (e.g. BER-EP4, CK7 and CK8/18; see also below). The normal myoepithelial cell is regarded to be negative for CK8/18, whilst many neoplastic myoepithelial cells are in fact positive for low molecular weight cytokeratins. The immunoprofile of modified neoplastic myoepithelial cells varies between the morphological subtypes in different tumours, and the expression also varies significantly from that of a myoepithelial cell in normal salivary gland tissue (see Chaps. 3 and 4). In normal salivary gland tissue, the myoepithelial cells surrounding the acini and intercalated ducts are positive for S-100, SMM and SMA, where S-100 often is patchy and SMA usually is weaker than SMM. The interpretation of vimentin and CD10 is obscured by positive staining of fibrous tissue around acini and ductal structures, whilst p63 gives a crisp nuclear staining, however, also of basal cells around striated and excretory ducts (Fig. 1.3). As mentioned above there are fundamental differences between the myoepithelial and the basal cells. Studies performed in relation to salivary regeneration, metaplasia and sialadenitis by Ihrler and associates have significantly contributed to our current knowledge of myoepithelial, basal, ductal and acinar cells [30–34]. The myoepithelial cells are thus characterised by co-expression of basal type keratin CK14, α-actin, p63, S-100 but not bcl-2, whilst the basal cells demonstrate co-expression of CK14, CK18.12, p63 and bcl-2 but not S-100. The myoepithelial cells are mainly restricted to acini and intercalated ducts and rarely occur in the striated ducts. The basal cells are found primarily in the striated and excretory ducts and not in the intercalated duct or around the acini. The myoepithelial cells represent a presumably long-living epithelial-mesenchymal hybrid with functional specialisation for the propulsion of saliva. The basal cells have only recently been accepted as a special cell type with a putative reserve cell function and the salivary duct with three distinctly composed segments, hence differ profoundly from ducts of other glands, e.g. the breast and prostate [33]. The p63 and p73 genes (members of the p53 family) are expressed in both the basal and myoepithelial cells and, in contrast to p53, play important roles in stem cell identity and cellular differentiation. Immunohistochemical staining of the p63 protein is a more reliable marker of the myoepithelial cell than is that of p73 [54].
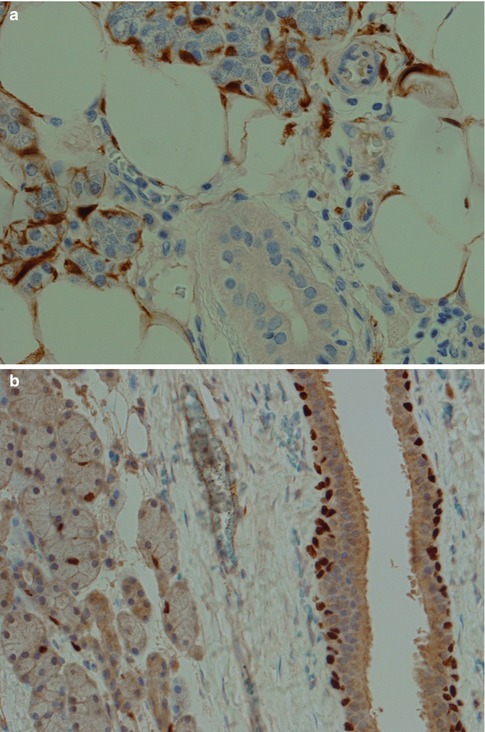

Fig. 1.3
Myoepithelial cells in normal salivary gland tissue. (a) Positive S-100 myoepithelial cells around acini and intercalated ducts but absent around striated ducts. (b) p63 staining of basal cells of an excretory duct and of myoepithelial cells surrounding acini
All ducts stain positive for most cytokeratins apart from CK20. The serous acinar cells stain with low molecular weight cytokeratins (CK8/18), CK18 and BER-EP4, and in most cases also for amylase, but are negative for EMA and high molecular weight cytokeratins (34βE12). The staining for CK7 and CEA varies, but it is predominantly CK7 positive and CEA negative. CEA may show luminal positivity. The serous acinar cells are also typically negative for CK20 and CK5/6. S-100 is strangely enough occasionally positive (10 %) (Fig. 1.4). The mucinous acinar cells seen in, for example, the submandibular gland, are negative for CK7 as well as for both low and high molecular weight cytokeratins, BER-EP4, CEA and EMA but surrounding basal cells stain positive for, e.g. CK14 (Fig. 1.5).
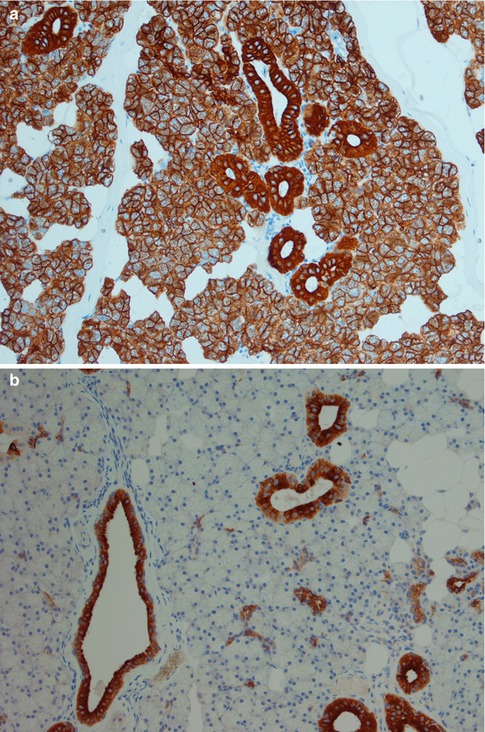
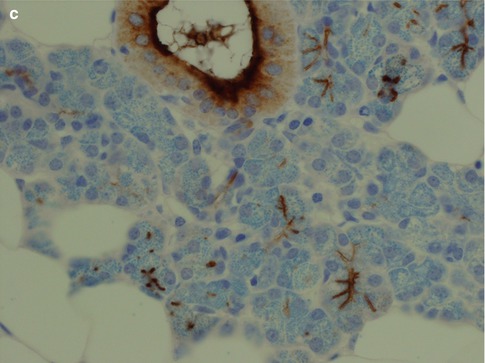
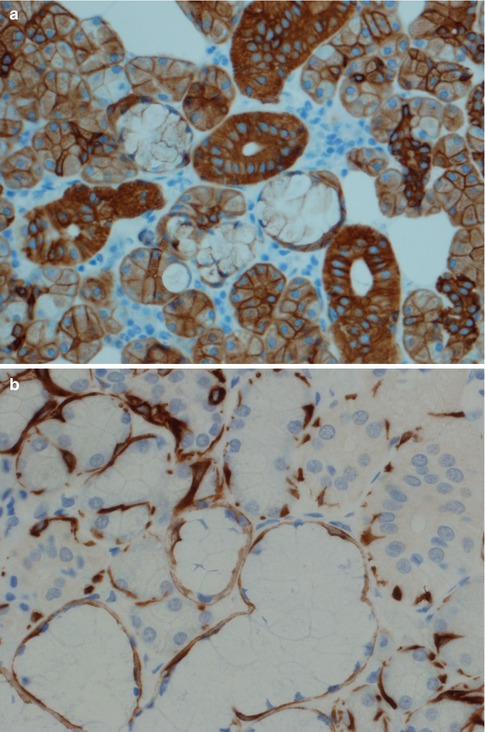






Fig. 1.4
(a) Both serous acinar cells and ducts stain positive cytokeratin CK8/18. (b) Serous acinar cells are negative for CK5/6 whilst the ducts are positive. (c) Similarly to CK5/6 the serous acinar cells are negative for EMA apart from luminal staining of the acini

Fig. 1.5
(a) Mucinous acinar cells are negative for CK7. (b) CK14 positivity in surrounding basal cells (mucinous cells centre-bottom, serous cells top-left, and ducts top-right)
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


