Fig. 3.1
Triage algorithm for patients with suspected hepatocellular carcinoma
In most cases, the clinical presentation will suggest whether the patient is at risk for primary or metastatic liver cancer. Once it is established that there is sufficient evidence of hepatic tumor, the initial diagnostic work-up of the patient will require adequate and cost-effective tests to confirm the presence of malignancy and the cell type, and to provide sufficient information to design and begin a treatment plan based on current guidelines and algorithms. The diagnostic work-up will include complete medical history, social and family history, physical examination, laboratory testing, and, diagnostic imaging. Laboratory testing will include metabolic profile, liver function tests, complete blood count, clotting tests, and tumor markers, such as AFP. Initial diagnostic imaging may include Computed Tomography (CT), Ultrasound (US), Magnetic Resonance Imaging (MRI) including Functional MRI, and, Photon Emission Tomography (PET).
3.1.3 Screening for Hepatocellular Carcinoma
Screening for HCC in high risk individuals is recommended since early identification of small tumors has been reported to lead to improved survival [13, 14]. High risk individuals include hepatitis B carriers and those with cirrhosis caused by HCV, alcoholic liver disease, genetic hemochromatosis, primary biliary cirrhosis, and autoimmune hepatitis [1] (Table 3.1).
Hepatitis B carriers (HBsAg positive) | Non hepatitis B carriers with cirrhosis |
|---|---|
Anyone with cirrhosis | Hepatitis C |
Asian males > 40 years | Primary biliary cirrhosis |
Asian females > 50 years | Alcoholic cirrhosis |
Africans > 20 years | Genetic Hemochromatosis |
Any family history of HCC | Alpha− 1- antitrypsin deficiency (possibly) |
Long term HBV carriers who lose HBsAg/develop anti-HBs | Cirrhosis from any other cause |
US has been recommended as a noninvasive imaging modality for screening for HCC; with a > 60 % sensitivity and > 90 % specificity in detecting HCC [15]. However, there may be considerable variations in detection rates based on operator experience, body habitus, the use of contrast agents, the size of liver mass, and the presence of cirrhosis [16]. Diagnostic features of HCC on US include irregular margins and internal echoes; however, smaller lesions can be hypoechoic [17]. Due to its low cost and wide availability, this modality is often used for screening in high risk patients. Screening is generally performed by experienced personnel in these patients at 6 month intervals with an US of liver [18]. Shorter follow-up interval (every 3–4 months) is recommended when a nodule of less than 1 cm has been detected and as a follow-up strategy for patients who have undergone resection or locoregional therapies [18].
AFP is a serologic marker that is elevated in many patients with HCC and is usually diagnostic in patients with at serum levels > 500 mcg/L [19]. However, AFP may also be elevated in pregnancy, those with HCV, cirrhosis without evidence of tumor [20], and in acute and chronic hepatitis [21]. In addition, serum AFP levels may also be normal in patients with a small HCC [22]. With a cutoff value of 25 ng/ml the sensitivity and specificity of AFP in diagnosing HCC has been reported as 69 % and 87 % respectively [23]. Given the limited sensitivity and specificity, AFP should not be used alone as either a screening agent or diagnostic tool [24]; however, AFP may be helpful in making a diagnosis of HCC in conjunction with other imaging modalities. Analysis of recent studies shows that AFP determination lacks adequate sensitivity and specificity for effective surveillance (and for diagnosis) [25, 26].
Another biomarker, des-gamma-carboxy prothrombin (DCP), is a prothrombin protein measured in the serum that is increased in patients with HCC [27]. DCP is thought to be a result of defective post-translational carboxylation that is found in patients with HCC [28]. A recent study in a Western population comparing AFP and DCP showed that DCP was more sensitive and specific in differentiation HCC from benign chronic liver disease [27]. However, in a more recent study, DCP did not offer substantial advantages with respect to AFP [28]. In addition, DCP levels have been associated to portal vein invasion and advanced tumor stage, a fact that prevents the usage of this marker for early detection [18, 29].
3.1.4 Diagnosis of Hepatocellular Carcinoma
Imaging plays an important role in confirming the diagnosis of HCC, and is discussed in greater detail in Chap. 4A. It is essential that diagnostic testing be performed properly according to established protocols so that errors in diagnosis are avoided, and so that the full extent of disease is established. The basic features in diagnostic liver imaging will include: (1) the size and number of hepatic lesions; (2) the location of these lesions with respect to segmental anatomy and relationship to adjacent intrinsic and extrinsic structures, including vasculature, diaphragm, structures of the GI tract; (3) enhancement characteristics on CT and/or MRI; (4) metabolic activity on PET scan and/or functional MRI studies; and, (5) the presence or absence of extra-hepatic disease or portal vein involvement.
Images, and at times 3-D reconstructions, should be available to the treating physicians so that specific anatomical relationships can be fully evaluated when precise treatment plans are being formulated.
Lesion size has a major impact on the evaluation and implication of findings on CT and MRI imaging.
3.1.4.1 Liver Lesions > 2 cm in Diameter
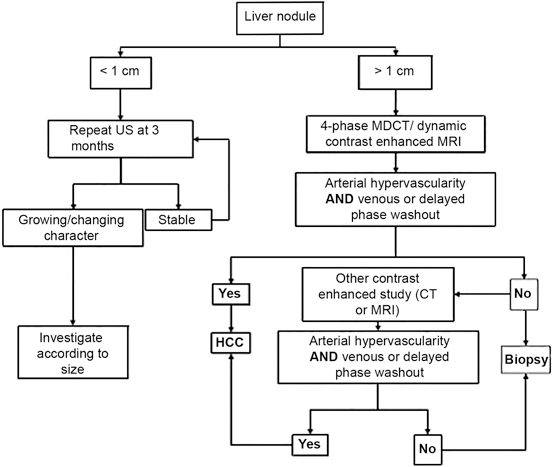
In the setting of a cirrhotic liver, a lesion that measures > 2 cm and has radiographic characteristics of HCC (arterial enhancement and portal phase washout) on a dynamic imaging study such as CT or MRI, liver biopsy is not required [18, 25, 26, 30]. In addition, if serum AFP measurement > 200 ng/ml and the mass seen on CT or MRI scan has features of HCC, a biopsy of liver lesion is also not necessary. Biopsy should be obtained in all lesions > 2 cm without a characteristic vascular enhancement profile or if the lesion occurs in a non-cirrhotic liver [18, 25, 26].
3.1.4.2 Liver Lesions 1–2 cm in Diameter
Lesions measuring 1–2 cm in a cirrhotic liver may be treated as HCC if they exhibit characteristic arterial hypervascularity with washout on two imaging modalities (CT, MRI or contrast US). However, if this pattern is not observed in both imaging studies a biopsy of liver lesions should be considered [18, 25, 26].
3.1.4.3 Liver Lesions < 1 cm in Diameter
Liver nodules measuring < 1 cm should be monitored with US imaging at 3–6 month intervals. If no growth is seen in 2 years, no action is needed and routine US surveillance is recommended [18, 25, 26].
An imaging algorithm for the diagnosis of HCC (Fig. 3.2) has been described by the American Association for the Study of Liver Diseases (AASLD) [25].
Liver biopsy is not recommended routinely for lesions > 2 cm that have imaging characteristics compatible with HCC in patients with cirrhosis and/or a serum a-fetoprotein level > 200 ng/mL. Confirmatory diagnostic testing, such as CT directed biopsy may still be required, especially when evaluation by imaging is inconclusive. However, it is important to note that the interpretation of biopsies and distinction between high-grade dysplastic nodules and HCC is challenging. Expert pathology diagnosis is reinforced by staining for glypican 3, Hsp 70, and glutamine synthetase, because positivity for two of these three stains confirms HCC [18, 25, 26].
Although, noninvasive imaging techniques for the diagnosis of HCC prior to liver transplantation has become accepted it should be kept in mind that in three recent studies there was a false positive rate of 9–30 % with no evidence of tumor in the explanted liver [3, 31, 32, 33]. This approach may have to be reconsidered if it is found that targeted therapy determined by tissue-based histopathological and/or molecular evaluation before treatment is feasible [3].
3.1.5 Staging of Hepatocellular Carcinoma
Staging in HCC differs from other solid tumors where prognosis and treatment are based primarily on tumor size and metastasis. In HCC, underlying liver function has a high prognostic importance and is integrated into many HCC staging systems. Given the complexity in diagnosing and prognosticating HCC, which usually occurs in the setting of poor liver function, many different staging systems including Tumor Node Metastasis (TNM) [34], Okuda staging system [35], Cancer of the Liver Italian Program (CLIP) [36, 37] and Barcelona Clinic Liver Cancer (BCLC) staging system [38] have been proposed that focus on various aspects of this disease. Although there is no consensus on which staging system is preferred, current American Association for the Study of Liver Diseases (AASLD), European Association for the Study of the Liver (AESL), and European Organisation for Research and Treatment of Cancer (EORTC) guidelines recommend the use of the BCLC system because it has been validated and integrates tumor stage, liver function, and physical status [18, 25, 26]. The TNM system classifies HCC based on the size of tumor, the number of nodules, and the presence of vascular invasion [39]. Since this staging system originally did not take into account underlying liver function, its use in predicting prognosis and guiding treatment was limited [25, 26]. An alternate version of the TNM staging system has been proposed that incorporates underlying liver histology and may be of greater clinical use. This system has been validated in patients undergoing surgical resection [40] and more recently it has been shown to accurately stratify patients after liver transplantation [41].
Similar to the TNM classification system, the Okuda system accounts for tumor size; however, it differs in that it does not take into account the presence of lymph node involvement and metastasis, and includes liver function parameters such as ascites, bilirubin, and serum albumin to stratify patients [35]. Although, the Okuda system is very useful in identifying patients with end stage HCC, it is limited in accurately differentiating patients with early and intermediate stage disease [25, 26].
The CLIP score is a mathematical score based on tumor morphology, presence or absence of portal vein thrombosis, AFP levels, and Child-Pugh class [37]. This simple scoring system has been shown to predict survival better than the TNM and Okuda systems [36, 42]; however, this system does not adequately stratify patients who may be candidates for resection or transplantation [16].
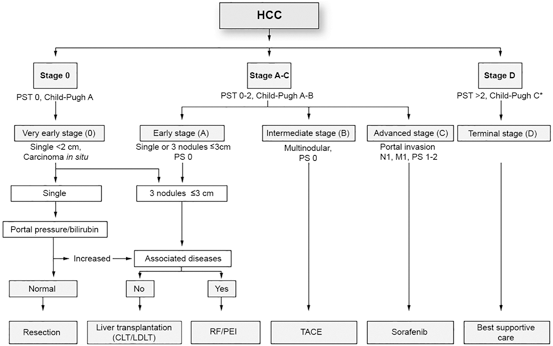
The BCLC classification system is based on performance status, extent of tumor including presence or absence of vascular invasion, bilirubin level, presence or absence of portal hypertension, and Okuda stage [38] (Fig. 3.3). This system is advantageous in that it classifies patients as very early, early, intermediate, advanced or terminal stages thereby establishing a link between stage of disease and appropriate treatment modalities [18, 25, 26]. Very early stage disease is difficult to diagnose since patients are Child-Pugh class A, have no clinical features of liver disease, and have a single HCC lesion < 2 cm. If diagnosis is made at this stage, the treatment of the tumor has a theoretical 5-year survival rate of 100 % [18, 25, 26]. Early stage disease includes patients with up to three nodules, each less than 3 cm, and well preserved liver function (Child-Pugh class A and B).