• Thalassemias, which are diseases that result from the decreased abundance of one or more of the globin chains (Case 44). The decrease can result from decreased production of a globin chain or, less commonly, from a structural variant that destabilizes the chain. The resulting imbalance in the ratio of the α:β chains underlies the pathophysiology of these conditions. Example: promoter mutations that decrease expression of the β-globin mRNA to cause β-thalassemia.
• Hereditary persistence of fetal hemoglobin, a group of clinically benign conditions that impair the perinatal switch from γ-globin to β-globin synthesis. Example: a deletion, found in African Americans, that removes both the δ- and β-globin genes but leads to continued postnatal expression of the γ-globin genes, to produce Hb F, which is an effective oxygen transporter (see Fig. 11-3).
Hemoglobin Structural Variants
Most variant hemoglobins result from point mutations in one of the globin structural genes. More than 400 abnormal hemoglobins have been described, and approximately half of these are clinically significant. The hemoglobin structural variants can be separated into the following three classes, depending on the clinical phenotype (Table 11-3):
• Variants that cause hemolytic anemia, most commonly because they make the hemoglobin tetramer unstable.
• Variants with altered oxygen transport, due to increased or decreased oxygen affinity or to the formation of methemoglobin, a form of globin incapable of reversible oxygenation.
• Variants due to mutations in the coding region that cause thalassemia because they reduce the abundance of a globin polypeptide. Most of these mutations impair the rate of synthesis of the mRNA or otherwise affect the level of the encoded protein.
Hemolytic Anemias
Hemoglobins with Novel Physical Properties: Sickle Cell Disease.
Sickle cell hemoglobin is of great clinical importance in many parts of the world. The disease results from a single nucleotide substitution that changes the codon of the sixth amino acid of β-globin from glutamic acid to valine (GAG → GTG: Glu6Val; see Table 11-3). Homozygosity for this mutation is the cause of sickle cell disease (Case 42). The disease has a characteristic geographical distribution, occurring most frequently in equatorial Africa and less commonly in the Mediterranean area and India and in countries to which people from these regions have migrated. Approximately 1 in 600 African Americans is born with this disease, which may be fatal in early childhood, although longer survival is becoming more common.
Clinical Features.
Sickle cell disease is a severe autosomal recessive hemolytic condition characterized by a tendency of the red blood cells to become grossly abnormal in shape (i.e., take on a sickle shape) under conditions of low oxygen tension (see Fig. 11-5). Heterozygotes, who are said to have sickle cell trait, are generally clinically normal, but their red cells can sickle when they are subjected to very low oxygen pressure in vitro. Occasions when this occurs are uncommon, although heterozygotes appear to be at risk for splenic infarction, especially at high altitude (for example in airplanes with reduced cabin pressure) or when exerting themselves to extreme levels in athletic competition. The heterozygous state is present in approximately 8% of African Americans, but in areas where the sickle cell allele (βS) frequency is high (e.g., West Central Africa), up to 25% of the newborn population are heterozygotes.
The Molecular Pathology of Hb S.
Nearly 60 years ago, Ingram discovered that the abnormality in sickle cell hemoglobin was a replacement of one of the 146 amino acids in the β chain of the hemoglobin molecule. All the clinical manifestations of sickle cell hemoglobin are consequences of this single change in the β-globin gene. Ingram’s discovery was the first demonstration in any organism that a mutation in a structural gene could cause an amino acid substitution in the corresponding protein. Because the substitution is in the β-globin chain, the formula for sickle cell hemoglobin is written as α2β2S or, more precisely, α2Aβ2S. A heterozygote has a mixture of the two types of hemoglobin, A and S, summarized as α2Aβ2A/α2Aβ2S, as well as a hybrid hemoglobin tetramer, written as α2AβAβS. Strong evidence indicates that the sickle mutation arose in West Africa but that it also occurred independently elsewhere. The βS allele has attained high frequency in malarial areas of the world because it confers protection against malaria in heterozygotes (see Chapter 9).
Sickling and Its Consequences.
The molecular and cellular pathology of sickle cell disease is summarized in Figure 11-6. Hemoglobin molecules containing the mutant β-globin subunits are normal in their ability to perform their principal function of binding oxygen (provided they have not polymerized, as described next), but in deoxygenated blood, they are only one fifth as soluble as normal hemoglobin. Under conditions of low oxygen tension, this relative insolubility of deoxyhemoglobin S causes the sickle hemoglobin molecules to aggregate in the form of rod-shaped polymers or fibers (see Fig. 11-5). These molecular rods distort the α2β2S erythrocytes to a sickle shape that prevents them from squeezing single file through capillaries, as do normal red cells, thereby blocking blood flow and causing local ischemia. They may also cause disruption of the red cell membrane (hemolysis) and release of free hemoglobin, which can have deleterious effects on the availability of vasodilators, such as nitric oxide, thereby exacerbating the ischemia.
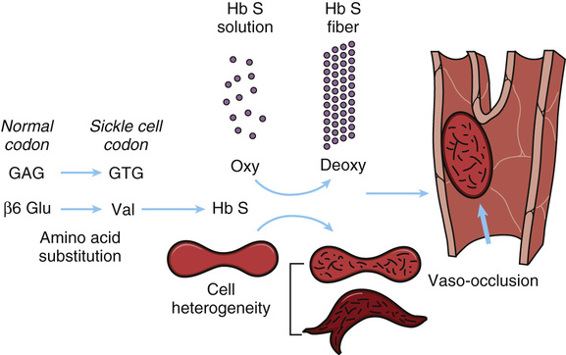
Modifier Genes Determine the Clinical Severity of Sickle Cell Disease.
It has long been known that a strong modifier of the clinical severity of sickle cell disease is the patient’s level of Hb F (α2γ2), higher levels being associated with less morbidity and lower mortality. The physiological basis of the ameliorating effect of Hb F is clear: Hb F is a perfectly adequate oxygen carrier in postnatal life and also inhibits the polymerization of deoxyhemoglobin S.
Until recently, however, it was not certain whether the variation in Hb F expression was heritable. Genome-wide association studies (GWAS) (see Chapter 10) have demonstrated that single nucleotide polymorphisms (SNPs) at three loci—the γ-globin gene and two genes that encode transcription factors, BCL11A and MYB—account for 40% to 50% of the variation in the levels of Hb F in patients with sickle cell disease. Moreover, the Hb F–associated SNPs are also associated with the painful clinical episodes thought to be due to capillary occlusion caused by sickled red cells (Fig. 11-6).
The genetically driven variations in the level of Hb F are also associated with variation in the clinical severity of β-thalassemia (discussed later) because the reduced abundance of β-globin (and thus of Hb A [α2β2]) in that disease is partly alleviated by higher levels of γ-globin and thus of Hb F (α2γ2). The discovery of these genetic modifiers of Hb F abundance not only explains much of the variation in the clinical severity of sickle cell disease and β-thalassemia, but it also highlights a general principle introduced in Chapter 8: modifier genes can play a major role in determining the clinical and physiological severity of a single-gene disorder.
BCL11A, a Silencer of γ-Globin Gene Expression in Adult Erythroid Cells.
The identification of genetic modifiers of Hb F levels, particularly BCL11A, has great therapeutic potential. The product of the BCL11A gene is a transcription factor that normally silences γ-globin expression, thus shutting down Hb F production postnatally. Accordingly, drugs that suppress BCL11A activity postnatally, thereby increasing the expression of Hb F, might be of great benefit to patients with sickle cell disease and β-thalassemia (see Chapter 13), disorders that affect millions of individuals worldwide. Small molecule screening programs to identify potential drugs of this type are now underway in many laboratories.
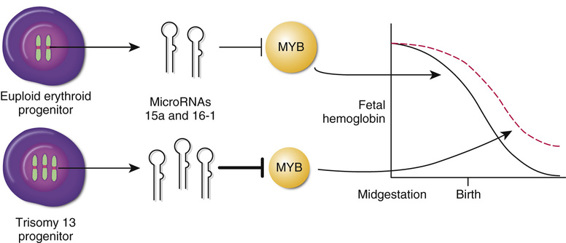
Trisomy 13, MicroRNAs, and MYB, Another Silencer of γ-Globin Gene Expression.
The indication from GWAS that MYB is an important regulator of γ-globin expression has received further support from an unexpected direction, studies investigating the basis for the persistent increased postnatal expression of Hb F that is observed in patients with trisomy 13 (see Chapter 6). Two miRNAs, miR-15a and miR-16-1, directly target the 3′ untranslated region (UTR) of the MYB mRNA, thereby reducing MYB expression. The genes for these two miRNAs are located on chromosome 13; their extra dosage in trisomy 13 is predicted to reduce MYB expression below normal levels, thereby partly relaxing the postnatal suppression of γ-globin gene expression normally mediated by the MYB protein, and leading to increased expression of Hb F (Fig. 11-7).
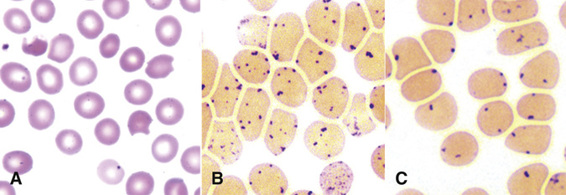
Unstable Hemoglobins.
The unstable hemoglobins are due largely to point mutations that cause denaturation of the hemoglobin tetramer in mature red blood cells. The denatured globin tetramers are insoluble and precipitate to form inclusions (Heinz bodies) that contribute to damage of the red cell membrane and cause the hemolysis of mature red blood cells in the vascular tree (Fig. 11-8, showing a Heinz body due to β-thalassemia).
The amino acid substitution in the unstable hemoglobin Hb Hammersmith (β-chain Phe42Ser; see Table 11-3) leads to denaturation of the tetramer and consequent hemolysis. This mutation is particularly notable because the substituted phenylalanine residue is one of the two amino acids that are conserved in all globins in nature (see Fig. 11-2). It is therefore not surprising that substitutions of this phenylalanine produce serious disease. In normal β-globin, the bulky phenylalanine wedges the heme into a “pocket” in the folded β-globin monomer. Its replacement by serine, a smaller residue, creates a gap that allows the heme to slip out of its pocket. In addition to its instability, Hb Hammersmith has a low oxygen affinity, which causes cyanosis in heterozygotes.
In contrast to mutations that destabilize the tetramer, other variants destabilize the globin monomer and never form the tetramer, causing chain imbalance and thalassemia (see following section).
Variants with Altered Oxygen Transport
Mutations that alter the ability of hemoglobin to transport oxygen, although rare, are of general interest because they illustrate how a mutation can impair one function of a protein (in this case, oxygen binding and release) and yet leave the other properties of the protein relatively intact. For example, the mutations that affect oxygen transport generally have little or no effect on hemoglobin stability.
Methemoglobins.
Oxyhemoglobin is the form of hemoglobin that is capable of reversible oxygenation; its heme iron is in the reduced (or ferrous) state. The heme iron tends to oxidize spontaneously to the ferric form and the resulting molecule, referred to as methemoglobin, is incapable of reversible oxygenation. If significant amounts of methemoglobin accumulate in the blood, cyanosis results. Maintenance of the heme iron in the reduced state is the role of the enzyme methemoglobin reductase. In several mutant globins (either α or β), substitutions in the region of the heme pocket affect the heme-globin bond in a way that makes the iron resistant to the reductase. Although heterozygotes for these mutant hemoglobins are cyanotic, they are asymptomatic. The homozygous state is presumably lethal. One example of a β-chain methemoglobin is Hb Hyde Park (see Table 11-3), in which the conserved histidine (His92 in Fig. 11-2) to which heme is covalently bound has been replaced by tyrosine (His92Tyr).
Hemoglobins with Altered Oxygen Affinity.
Mutations that alter oxygen affinity demonstrate the importance of subunit interaction for the normal function of a multimeric protein such as hemoglobin. In the Hb A tetramer, the α:β interface has been highly conserved throughout evolution because it is subject to significant movement between the chains when the hemoglobin shifts from the oxygenated (relaxed) to the deoxygenated (tense) form of the molecule. Substitutions in residues at this interface, exemplified by the β-globin mutant Hb Kempsey (see Table 11-3), prevent the normal oxygen-related movement between the chains; the mutation “locks” the hemoglobin into the high oxygen affinity state, thus reducing oxygen delivery to tissues and causing polycythemia.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



