Hemodialysis and Peritoneal Dialysis
KEY CONCEPTS
![]() Hemodialysis (HD) involves the perfusion of blood and dialysate on opposite sides of a semipermeable membrane. Solutes are removed from the blood by diffusion and convection. Excess plasma water is removed by ultrafiltration.
Hemodialysis (HD) involves the perfusion of blood and dialysate on opposite sides of a semipermeable membrane. Solutes are removed from the blood by diffusion and convection. Excess plasma water is removed by ultrafiltration.
![]() Native arteriovenous (AV) fistulas are the preferred access for HD because of fewer complications and a longer survival rate. Venous catheters are plagued by complications such as infection and thrombosis and often deliver low blood flow rates.
Native arteriovenous (AV) fistulas are the preferred access for HD because of fewer complications and a longer survival rate. Venous catheters are plagued by complications such as infection and thrombosis and often deliver low blood flow rates.
![]() Adequacy of HD can be assessed by the Kt/V and urea reduction ratio (URR). The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative minimum goal Kt/V is greater than 1.2 per treatment and the URR is greater than 65%.
Adequacy of HD can be assessed by the Kt/V and urea reduction ratio (URR). The National Kidney Foundation’s Kidney Disease Outcomes Quality Initiative minimum goal Kt/V is greater than 1.2 per treatment and the URR is greater than 65%.
![]() During HD, patients commonly experience hypotension and cramps. Other more serious complications include infection and thrombosis of the vascular access.
During HD, patients commonly experience hypotension and cramps. Other more serious complications include infection and thrombosis of the vascular access.
![]() Peritoneal dialysis (PD) involves the instillation of dialysate into the peritoneal cavity via a permanent peritoneal catheter. The peritoneal membrane lines the highly vascularized abdominal viscera and acts as the semipermeable membrane. Solutes are removed from the blood across the peritoneum via diffusion and ultrafiltration. Excess plasma water is removed via ultrafiltration created by osmotic pressure generated by various dextrose or icodextrin concentrations.
Peritoneal dialysis (PD) involves the instillation of dialysate into the peritoneal cavity via a permanent peritoneal catheter. The peritoneal membrane lines the highly vascularized abdominal viscera and acts as the semipermeable membrane. Solutes are removed from the blood across the peritoneum via diffusion and ultrafiltration. Excess plasma water is removed via ultrafiltration created by osmotic pressure generated by various dextrose or icodextrin concentrations.
![]() Patients on PD are required to instill and drain, manually or via automated systems, several liters of fresh dialysate each day. The more exchanges completed each day results in greater solute removal.
Patients on PD are required to instill and drain, manually or via automated systems, several liters of fresh dialysate each day. The more exchanges completed each day results in greater solute removal.
![]() Peritonitis is a common complication of PD. Initial empiric therapy for peritonitis should include intraperitoneal (IP) antibiotics that are effective against both gram-positive and gram-negative organisms.
Peritonitis is a common complication of PD. Initial empiric therapy for peritonitis should include intraperitoneal (IP) antibiotics that are effective against both gram-positive and gram-negative organisms.
![]() Nasal carriage of Staphylococcus aureus is associated with an increased risk of catheter-related infections and peritonitis. Prophylaxis with intranasal mupirocin (twice a day for 5 days every month) or mupirocin (daily) at the exit site can effectively reduce S. aureus infections.
Nasal carriage of Staphylococcus aureus is associated with an increased risk of catheter-related infections and peritonitis. Prophylaxis with intranasal mupirocin (twice a day for 5 days every month) or mupirocin (daily) at the exit site can effectively reduce S. aureus infections.
INTRODUCTION
The three primary treatment options for patients with end-stage renal disease (ESRD) are hemodialysis (HD), peritoneal dialysis (PD), and kidney transplantation. The United States Renal Data System (USRDS) is the national system that “collects, analyzes, and distributes” data relating to patients with ESRD or Stage 5 chronic kidney disease (CKD) in the United States.1 According to the 2011 USRDS, at the end of 2009, there are more than 550,000 patients in the United States with ESRD. Of these, 370,274 and 27,522 patients were being treated with HD and PD, respectively, and 172,553 had a functioning kidney transplant. In 2009, 116,395 new patients started therapy for ESRD (dialysis or transplantation) and more than 91,000 patients died. The vast majority of new dialysis patients are treated with HD. The number of patients treated with PD has steadily decreased since 2000.1 Although the number of patients who have received a kidney transplant has risen, transplantation has not kept pace with the growing prevalence of ESRD in the United States.1
Since 1972, the treatment of ESRD (both dialysis and kidney transplantation) has been paid for by Medicare. The total cost of ESRD in 2009 was 42.5 billion dollars, this includes Medicare costs (29 billion), Medicare patient obligation costs (4.2 billion), and non-Medicare costs (9.3 billion). Total Medicare spending for ESRD in 2009 rose by 3.1%. The Medicare spending does not include Part D expenditures, which were 1.55 billion in 2008. ESRD consumes a vastly disproportionate amount of resources; approximately 1% of the patients in the Medicare program have ESRD, yet 6% of the budget is consumed by the ESRD program. Although total spending for ESRD treatment continues to climb, per-patient spending (after adjusting for inflation) was fairly flat recently.1
There are some positive signs as it relates to public health and ESRD. Although the total number of dialysis patients is increasing in the United States, the number of new dialysis patients per total population has stabilized or slightly decreased from the highest value observed in 1997. The prevalence of ESRD continues to climb, reflective of reduced mortality and enhanced patient care. The primary diagnosis for new patients with ESRD is diabetes.1 Chapter 29 provides a thorough discussion on the epidemiology of CKD.
This chapter serves as a primer on the principles and practice of dialysis and the complications associated with the delivery of dialysis treatments. The chapter focuses on HD and PD as the modalities most commonly employed for the management of ESRD (see Chap. 28 for a discussion of the role of renal replacement therapies in the management of acute kidney injury). The pertinent factors that should be considered before the initiation of dialysis are described. The morbidity and mortality associated with HD and PD are compared, as these considerations may influence the dialysis method chosen by patients and clinicians. Because dialysis by either method is not a generic procedure, the variants of HD and PD are detailed. The multiple types of vascular and peritoneal access used to provide HD and PD, including various catheters and surgical techniques, are illustrated. The concept of dialysis adequacy for each modality is briefly reviewed. Finally, the clinical presentation of the common complications of both dialytic therapies is presented, along with pertinent nonpharmacologic and pharmacologic therapeutic approaches. Patient-related videos that describe living with CKD, dialysis, and associated dialysis therapies are shown in Table 30-1. This information is included to provide the reader with a patient perspective into the disease and its associated therapies.
TABLE 30-1 Patient-Related Videos Relative to Dialysis Procedures and Therapies

MORBIDITY AND MORTALITY IN DIALYSIS PATIENTS
Morbidity in patients receiving dialysis can be assessed in a number of different ways including tabulation of the number of hospitalizations per patient-year, the number of days hospitalized per patient per year, or the incidence of certain complications. The number of all-cause hospital admissions in dialysis patients per patient-year (1.9 hospitalizations per patient-year) have changed little since 1993. However, the rate of hospitalizations fell in 2006, to a rate approximately 4% less than that in 1993. Trends in hospitalization demonstrate an increase in hospitalization as a consequence of infection and cardiovascular disease and a decrease in hospitalizations as a consequence of vascular access problems. Patients with a functioning kidney transplant have a lower rate of hospitalization and shorter length of stay. Hospitalizations are more frequent for whites than for blacks, and the frequency and duration increase with age in both dialysis modality groups.1
The life expectancy of U.S. dialysis patients is markedly lower than that of healthy subjects of the same age and sex. In those older than 65 years, the risk of dying is twofold higher in dialysis patients compared with those with diabetes, cancer, heart failure, and cardiovascular disease.1 Approximately 50% of deaths in dialysis patients are cardiovascular related. In fact, those with CKD are more likely to die from cardiovascular disease before they reach ESRD. Infections, usually related to the dialysis access, are the second most common cause of death in dialysis patients. Although mortality is high in this patient population, improvement has been made and the overall patient mortality rate has fallen among dialysis patients since 1988. The changes in mortality rates are more impressive when the duration of a patient’s time receiving dialysis is considered. In patients receiving dialysis for fewer than 2 years, mortality rates decreased 25% since 1988. However, in those treated for 5 years or more, mortality rates increased 10%. These changes suggest that death is occurring later in the course of dialysis therapy. Regardless, in the United States, nearly two thirds of all dialysis patients die within 5 years of initiation of dialysis treatment, a life expectancy worse than patients with heart failure or numerous cancers.2
In addition to the morbidity and mortality discussed above a dialysis patient’s quality of life is generally poor. Quality-of-life assessments including the impact of dialysis treatment on these patients have been an area of considerable research.3–6 Additionally, in an effort to understand how these patients manage the constraints and difficulties of their life situations health care providers and researchers have provided commentaries and papers.7–11 For example, restrictions caused by thrice weekly HD and/or associated treatments have been shown to impact many areas of a patient’s life. These include but are not limited to, physical endurance, sex life, employment, social life, and dietary restrictions. Patients often complain of fatigue and fear of the unknown related to their disease and its progression. Although, the authors of this chapter may be able to describe the disruption to a patient’s life induced by this chronic disease only a kidney disease patient can adequately describe the three trips per week to the outpatient HD unit for a 3- to 4-hour HD session. The PD patient or the home HD patient may have some freedom from these restrictions, but this freedom comes with its own constraints (see the videos listed in Table 30-1).
INDICATIONS FOR DIALYSIS
The National Kidney Foundation’s Kidney Disease Outcome Quality Initiative (NKF-K/DOQI) recommends that planning for dialysis begin when patients reach CKD stage 4 (estimated glomerular filtration rate [eGFR] or creatinine clearance [CLcr] below 30 mL/min per 1.73 m2 [0.29 mL/s/m2]).12 Beginning the preparation process at this point allows adequate time for proper education of the patient and family and for the creation of a suitable vascular or peritoneal access. For patients choosing HD, a permanent arteriovenous (AV) access (preferably a fistula) should be surgically created when Clcr or eGFR falls below 25 mL/min (0.42 mL/s), serum creatinine is greater than 4 mg/dL (354 μmol/L), or 1 year prior to the anticipated need for dialysis.13
The primary criterion for initiation of dialysis is the patient’s clinical status: the presence of persistent anorexia, nausea, and vomiting, especially if accompanied by weight loss, fatigue, declining serum albumin concentrations, uncontrolled hypertension or congestive heart failure, and neurologic deficits or pruritus. Some nephrologists use critical lab values of serum creatinine or blood urea nitrogen as indicators of when to initiate dialysis. The 2006 update of the NKF-K/DOQI guidelines suggest that risks and benefits of dialysis should be evaluated when eGFR or CLcr is <15 mL/min per 1.73 m2 (<0.14 mL/s/m2).12,14 The advantages and disadvantages of HD and PD are depicted in Tables 30-2 and 30-3, respectively. These factors, along with the patients’ concomitant diseases, personal preferences, and support environments, are the principal determinants of the dialysis mode they will receive.2
TABLE 30-2 Advantages and Disadvantages of Hemodialysis
TABLE 30-3 Advantages and Disadvantages of Peritoneal Dialysis

Clinical Controversy…
While the intent of this chapter is not to exhaustively compare and contrast HD and PD and the relative benefits of each, there is considerable debate in the literature regarding the mortality differences between HD and PD. A recent trial examining mortality in dialysis patients in the Netherlands found no difference between patients receiving either modality in the first 2 years. However after that mortality rates were higher in patients on PD.14 Most observational trials suggest that PD is associated with a survival advantage early in therapy, which wanes with increased treatment time. Well-designed studies are extremely difficult to conduct in this population and thus the question of superiority of one modality over the other is controversial. Differences in outcomes may be related to a wide array of confounding factors, such as the dose of dialysis, baseline patient health status, physician bias in modality selection, patient compliance with dialysis and medication therapy, or other unknown factors. For example, healthier patients tend to be directed toward PD and factors such as age, duration of dialysis, and comorbidities play an important role in the complex relationship between patient outcomes and mortality.15,16 Without clear distinction between modalities in terms of many important outcomes, the selection of the optimal therapy for a given patient is challenging. The NKF-K/DOQI guidelines recommend that the timing of dialysis initiation is a compromise between maximizing patient QOL by extending the dialysis-free period while avoiding complications that will decrease the length and quality of dialysis-assisted life.12
HEMODIALYSIS
Although HD was first successfully used in 1940, the procedure was not used widely until the Korean War in 1952. Permanent dialysis access was developed in the 1960s,17 which allowed routine use of HD in patients with ESRD. Subsequent decades brought advances in dialysis technology, including the introduction of more efficient and biocompatible dialyzer membranes and safer techniques. HD is now the most common type of renal replacement therapy for patients with ESRD.
Principles of Hemodialysis
![]() HD, simply stated, consists of the perfusion of blood and a physiologic solution on opposite sides of a semipermeable membrane.18 Multiple substances, such as water, urea, creatinine, uremic toxins, and drugs, move from the blood into the dialysate, by either passive diffusion or convection as the result of ultrafiltration. Diffusion is the movement of substances down a concentration gradient; usually for endogenous waste products from the blood to dialysate; the rate of diffusion depends on the difference between the concentration of the solute in blood and dialysate, solute characteristics, that is size, water solubility, and charge, the dialyzer membrane composition, and blood and dialysate flow rates. Diffusive transport is rapid for small solutes, but slows with increasing molecular size. Other important diffusive solute transport factors include the membrane thickness, porosity, and the steric hindrance between the membrane pores and solute. Ultrafiltration is the movement of water across the dialyzer membrane as a consequence of hydrostatic or osmotic pressure and is the primary means for removal of excess fluid. Convection occurs when dissolved solutes are “dragged” across a membrane with fluid transport (if the pores in the dialyzer are large enough to allow them to pass). Convection can be maximized by increasing the hydrostatic pressure gradient across the dialysis membrane, or by changing to a dialyzer that is more permeable to water transport. These two processes of diffusion and convection can be controlled independently, and thus a patient’s HD prescription can be individualized to attain the desired degree of solute and fluid removal.18
HD, simply stated, consists of the perfusion of blood and a physiologic solution on opposite sides of a semipermeable membrane.18 Multiple substances, such as water, urea, creatinine, uremic toxins, and drugs, move from the blood into the dialysate, by either passive diffusion or convection as the result of ultrafiltration. Diffusion is the movement of substances down a concentration gradient; usually for endogenous waste products from the blood to dialysate; the rate of diffusion depends on the difference between the concentration of the solute in blood and dialysate, solute characteristics, that is size, water solubility, and charge, the dialyzer membrane composition, and blood and dialysate flow rates. Diffusive transport is rapid for small solutes, but slows with increasing molecular size. Other important diffusive solute transport factors include the membrane thickness, porosity, and the steric hindrance between the membrane pores and solute. Ultrafiltration is the movement of water across the dialyzer membrane as a consequence of hydrostatic or osmotic pressure and is the primary means for removal of excess fluid. Convection occurs when dissolved solutes are “dragged” across a membrane with fluid transport (if the pores in the dialyzer are large enough to allow them to pass). Convection can be maximized by increasing the hydrostatic pressure gradient across the dialysis membrane, or by changing to a dialyzer that is more permeable to water transport. These two processes of diffusion and convection can be controlled independently, and thus a patient’s HD prescription can be individualized to attain the desired degree of solute and fluid removal.18
Hemodialysis Access
![]() Obtaining and maintaining access to the circulation has been a challenge for long-term use and success of HD.18 The AV fistula, AV graft, or venous catheter through which blood is obtained for dialysis is referred to as the dialysis access. Permanent access to the circulation may be accomplished by several techniques, including the creation of an AV fistula, an AV graft, or by the use of venous catheters (Fig. 30-1).19 The native AV fistula is created by the anastomosis of a vein and artery (i.e., the radial artery to the cephalic vein or the brachial artery to the cephalic vein). The native AV fistula has many advantages over other access methods. Fistulas have the longest survival of all blood-access devices and are associated with the lowest rate of complications such as infection and thrombosis. In addition, patients with fistulas have increased survival and lower hospitalization rates compared to other HD patients. Finally, the use of AV fistulas is the most cost-effective in terms of placement and long-term maintenance. Ideally, the most distal site (the wrist) is used to construct the fistula. This fistula is the easiest to create, and in the case of access failure, more proximal sites on the arm are preserved. Unfortunately, fistulas require 1 to 2 months or more to mature before they can be routinely utilized for dialysis. In addition, creation of an AV fistula may be difficult in elderly patients and in patients with peripheral vascular disease (which is particularly common in patients with diabetes).
Obtaining and maintaining access to the circulation has been a challenge for long-term use and success of HD.18 The AV fistula, AV graft, or venous catheter through which blood is obtained for dialysis is referred to as the dialysis access. Permanent access to the circulation may be accomplished by several techniques, including the creation of an AV fistula, an AV graft, or by the use of venous catheters (Fig. 30-1).19 The native AV fistula is created by the anastomosis of a vein and artery (i.e., the radial artery to the cephalic vein or the brachial artery to the cephalic vein). The native AV fistula has many advantages over other access methods. Fistulas have the longest survival of all blood-access devices and are associated with the lowest rate of complications such as infection and thrombosis. In addition, patients with fistulas have increased survival and lower hospitalization rates compared to other HD patients. Finally, the use of AV fistulas is the most cost-effective in terms of placement and long-term maintenance. Ideally, the most distal site (the wrist) is used to construct the fistula. This fistula is the easiest to create, and in the case of access failure, more proximal sites on the arm are preserved. Unfortunately, fistulas require 1 to 2 months or more to mature before they can be routinely utilized for dialysis. In addition, creation of an AV fistula may be difficult in elderly patients and in patients with peripheral vascular disease (which is particularly common in patients with diabetes).
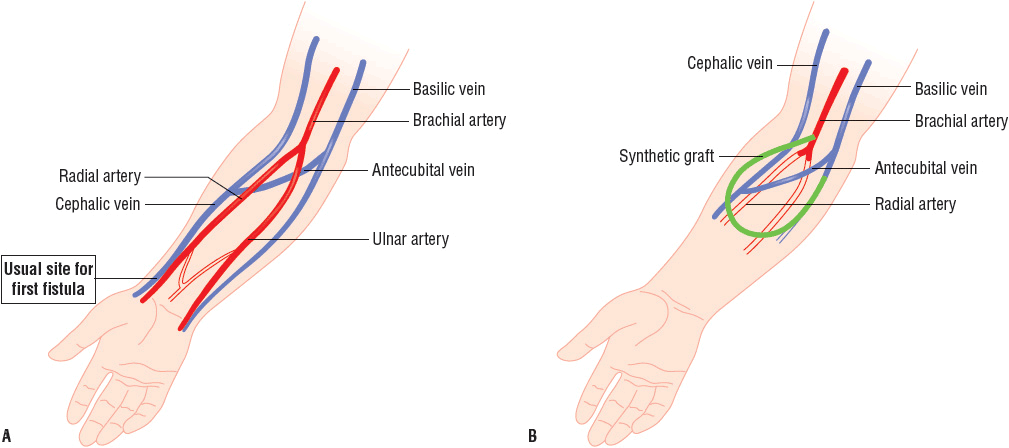
FIGURE 30-1 The predominant types of vascular access for chronic dialysis patients are (A) the AV fistula and (B) the synthetic AV forearm graft. The first primary AV fistula is usually created by the surgical anastomosis of the cephalic vein with the radial artery. The flow of blood from the higher-pressure arterial system results in hypertrophy of the vein. The most common AV graft (depicted in green) is between the brachial artery and the basilic or cephalic vein. The flow of blood may be diminished in the radial and ulnar arteries since it preferentially flows into the low pressure graft.
Synthetic AV grafts, usually made of polytetrafluoroethylene, are another option for permanent AV access. In general, grafts require only 2 to 3 weeks to endothelialize before they can be routinely used. The primary disadvantages of this type of access when compared to an AV fistula are shorter survival of the graft, and higher rates of infection and thrombosis. The least-desirable HD access is via central venous catheters (CVCs), which, unfortunately, are commonly used in chronic HD patients. Venous catheters can be placed in the femoral, subclavian, or internal jugular vein. The main advantage of catheters is that they can be used immediately. Catheters are often used in small children, diabetic patients with severe vascular disease, the morbidly obese, and patients who have no viable sites for permanent AV access. Late referrals to a nephrology specialist and delayed placement of a more appropriate long-term access contribute to the overuse of venous catheters in chronic HD patients. The major problem with all venous catheters is that they have a short life span and are more prone to infection and thrombosis than either AV grafts or fistulas. Furthermore, some catheters are not able to provide adequate blood flow rates, which can limit the dose of dialysis delivered.19–23 Regardless, tunneled dialysis catheters are used frequently for a variety of reasons including ease of insertion, pain-free dialysis, and immediate use. They are however associated with increased morbidity, mortality, and increased cost.24
The ESRD Clinical Performance Measures (CPM) Project examined quality of dialysis care markers, including anemia management, serum albumin, vascular access (for HD), and adequacy of dialysis. The report evaluated a sample population of adult in-center patients, 8,915 HD patients and 1,469 PD patients.25 At the end of 2005, 54% and 44% of incident and prevalent patients, respectively, were using AV fistulas for HD. The CPM Project’s goal is that 50% and 40% of incident and prevalent HD patients, respectively, should be using an AV fistula. Unfortunately, 21% of HD patients were using chronic catheters in 2005. This percent of patients using catheters is much higher than the CPM Project’s goal of <10%. The extensive use of catheters may be a result of the large population of patients who are not candidates for AV access, or that they are being used until permanent AV access can be accomplished. As noted earlier, timely referral to a nephrologist and vascular surgeon is necessary for the placement of the most appropriate access.
Hemodialysis Procedures
The HD system consists of an external vascular circuit through which the patient’s blood is transferred in sterile polyethylene tubing to the dialyzer via a mechanical pump (Fig. 30-2).26 The patient’s anticoagulated blood then passes through the dialyzer on one side of the semipermeable membrane and is returned to the patient. The dialysate solution, which consists of purified water and electrolytes, is pumped through the dialyzer countercurrent to the flow of blood on the opposite side of the semipermeable membrane. In most cases, systemic anticoagulation (with heparin) is used to prevent clotting of the HD circuit. The process of dialysis results in the removal of metabolic waste products and water and replenishment of body buffers.18 There are three broad categories of dialysis membranes: low flux, high efficiency, and high flux. Low-flux dialyzers, mostly made of cuprophane or cellulose acetate, have small pores that limit clearance to relatively small molecules (size ≤500 daltons) such as urea and creatinine. High-efficiency membranes have large surface areas and thus have a greater ability to remove water, urea, and other small molecules. High-flux membranes have larger pores that are capable of removing high-molecular-weight substances, such as β2-microglobulin, and vancomycin in addition to other larger molecular weight drugs.26,27 The primary reason to use high-efficiency and/or high-flux membranes is that clearance of both low- and high-molecular-weight substances is much greater than with the conventional membranes, allowing for shorter treatment times. To maximize the capacity or fully utilize the filter’s high flux membrane, high-efficiency and high-flux dialysis require blood flow rates greater than 400 mL/min, dialysate flow rates greater than 500 mL/min, and the use of strict controls on the rate of fluid removal. Typically these dialyzers are composed of polysulfone, polymethylmethacrylate, polyamide, cellulose triacetate, and polyacrylonitrile.26
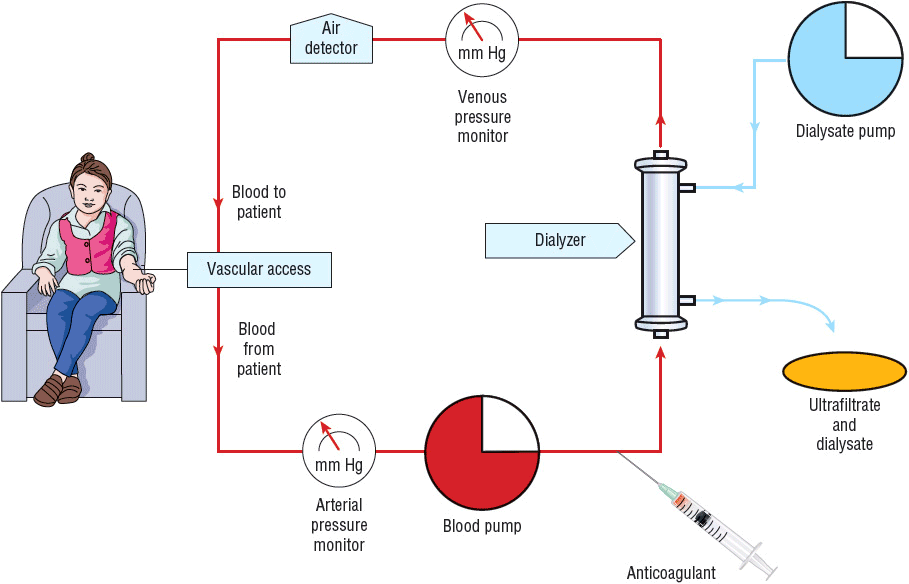
FIGURE 30-2 In HD, the patient’s blood is pumped to the dialyzer at a rate of 300 to 600 mL/min. An anticoagulant (usually heparin) is administered to prevent clotting in the dialyzer. The dialysate is pumped at a rate of 500 to 1,000 mL/min through the dialyzer countercurrent to the flow of blood. The rate of fluid removal from the patient is controlled by adjusting the pressure in the dialysate compartment.
HD is usually prescribed three times weekly for 3 to 5 hours. The average duration of dialysis treatment session in the United States in 2005 was just over 3.5 hours.25 Larger patients generally require longer treatment times for adequate solute removal. This is a substantial time commitment for any patient undergoing HD and results in substantial loss of control over their life. Other types of HD have been explored in an effort to balance dialysis adequacy with patient outcomes and quality of life. Short daily HD, extended dialysis, and quotidian HD are all terms used to describe variants of HD in which dialysis is administered daily for shorter periods of time (2 to 2.5 hours) or as long, slow nocturnal treatments of up to 6 to 8 hours. The theoretical rationale for these treatments is to enhance the efficiency of HD and to reduce HD-induced hemodynamic instability. There is some evidence that these dialysis techniques result in improved clinical outcomes and that it may be a more cost-effective dialysis procedure.28,29 Both of these therapeutic options are usually delivered in the home. The delivery of traditional HD in the home setting is more commonly used in some countries such as New Zealand and Canada. Despite the perceived advantages, the use of home HD is uncommon in the United States, with less than 1% of dialysis patients receiving HD care at home.1 Prospective clinical trials are needed in this area to elucidate the role of these types of dialysis therapy.
Measures of Hemodialysis Adequacy
The optimal dose of HD for each individual patient varies and is that the amount of therapy above which there is no cost-effective increment in the patient’s quality-adjusted life expectancy. The two primary goals of the dialysis prescription are to achieve a desired dry weight and the adequate removal of endogenous waste products such as urea. Dry weight is the target postdialysis weight at which the patient is normotensive and free of edema. Measurement of urea removal, while imperfect, is the typical way in which dialysis adequacy is quantified. Referred to as the “delivered dose” of dialysis, urea removal is utilized as the surrogate for removal of other toxins.
The delivered or desired dose of dialysis in terms of solute removal can be expressed as the URR or the Kt/V (pronounced “K-T-over-V”). The URR is a simple concept and is easily calculated as:
![]()
The URR is frequently used to measure the delivered dialysis dose, however, it does not account for the contribution of convective removal of urea. The Kt/V is a unitless index based on the dialyzer clearance of urea (K) in L/h multiplied by the duration of dialysis (t) in hours, divided by the urea distribution volume of the patient (V) in liters.30 Kt/V is thus the fraction of the patient’s total body water that is cleared of urea during a dialysis session. Urea kinetic modeling, using computer software, is the optimal means to calculate the Kt/V.31 An in-depth discussion of the pros and cons of various methods of calculating and interpreting Kt/V is beyond the scope of this chapter. The reader is referred to other sources for more in-depth information.30,31
![]() The NKF-K/DOQI recommends that the minimally adequate delivered dose of dialysis is a Kt/V of 1.2 (equivalent to an average URR of 65%).12 To achieve this goal, the recommended target prescribed Kt/V is 1.4 (equivalent to an average URR of 70%).12 Lower levels of dialysis treatment are thought to be associated with increased morbidity and mortality.18 Many nephrologists believe that even greater doses of dialysis would have positive outcomes in dialysis patients, and so the average dose of dialysis has been increasing in the United States. In 2004, the mean delivered Kt/V as reported by the CPM was 1.55.25 The Hemodialysis (HEMO) Study was designed to determine the effects of high-dose dialysis and the use of high-flux HD membranes on morbidity and mortality.32 The results of this prospective, randomized trial that assigned patients to either standard (Kt/V = 1.25) or high-dose (Kt/V = 1.65) dialysis with high-flux or low-flux membranes revealed that the risk of death was similar in both the standard and high-dose therapy and the low- and high-flux groups. Thus there does not appear to be any benefit in increasing the amount of dialysis above the current recommendations. Although many patients in the United States are well above the target Kt/V range, there is no reason to believe that nephrologists will begin to decrease their dose of dialysis. The HEMO study only enrolled patients who were on traditional thrice-weekly dialysis, thus the applicability of these findings to patients on more intensive regimens, such as daily or nocturnal HD regimens that provide long, frequent dialysis, remains to be determined,28,29,33 although early data indicate that these intensive HD regimens result in better blood pressure, anemia, and phosphate control.28 In those relatively few patients who are below the adequacy goal, the deficiency may be related to patient compliance with dialysis prescription (ending dialysis early) or low blood flow rates caused by access stenosis or thrombosis, or as a result of the use of catheters. Adequate dialysis may not be achieved in some patients despite compliance and sufficient blood flow. For these patients there are really only two options to increase urea clearance: use a larger membrane or increase the treatment time.
The NKF-K/DOQI recommends that the minimally adequate delivered dose of dialysis is a Kt/V of 1.2 (equivalent to an average URR of 65%).12 To achieve this goal, the recommended target prescribed Kt/V is 1.4 (equivalent to an average URR of 70%).12 Lower levels of dialysis treatment are thought to be associated with increased morbidity and mortality.18 Many nephrologists believe that even greater doses of dialysis would have positive outcomes in dialysis patients, and so the average dose of dialysis has been increasing in the United States. In 2004, the mean delivered Kt/V as reported by the CPM was 1.55.25 The Hemodialysis (HEMO) Study was designed to determine the effects of high-dose dialysis and the use of high-flux HD membranes on morbidity and mortality.32 The results of this prospective, randomized trial that assigned patients to either standard (Kt/V = 1.25) or high-dose (Kt/V = 1.65) dialysis with high-flux or low-flux membranes revealed that the risk of death was similar in both the standard and high-dose therapy and the low- and high-flux groups. Thus there does not appear to be any benefit in increasing the amount of dialysis above the current recommendations. Although many patients in the United States are well above the target Kt/V range, there is no reason to believe that nephrologists will begin to decrease their dose of dialysis. The HEMO study only enrolled patients who were on traditional thrice-weekly dialysis, thus the applicability of these findings to patients on more intensive regimens, such as daily or nocturnal HD regimens that provide long, frequent dialysis, remains to be determined,28,29,33 although early data indicate that these intensive HD regimens result in better blood pressure, anemia, and phosphate control.28 In those relatively few patients who are below the adequacy goal, the deficiency may be related to patient compliance with dialysis prescription (ending dialysis early) or low blood flow rates caused by access stenosis or thrombosis, or as a result of the use of catheters. Adequate dialysis may not be achieved in some patients despite compliance and sufficient blood flow. For these patients there are really only two options to increase urea clearance: use a larger membrane or increase the treatment time.
COMPLICATIONS OF CHRONIC KIDNEY DISEASE
Patients with CKD defined as a glomerular filtration rate (GFR) <60 mL/min/1.73 m2 (<0.58 mL/s/m2) are more likely to have at least one additional comorbid disease such as diabetes, hypertension, cardiovascular disease (CVD), BMI ≥30 kg/m2. CKD patients are also more likely to be older (age ≥60). The most recent NHANES III data (2005 to 2010) report the prevalence of these comorbid diseases in CKD patients as diabetes 19.3%, hypertension 14.8%, CVD 24.3%, and BMI ≥30 kg/m2 11.7% with older age at 18.4%. When compared with the NHANES III 1988 to 1994 report the 2005 to 2010 report found that the largest increase in CKD patients was among those patients with CVD (25.4% to 40.8%) although the prevalence of CKD patients only increased from 12.3% to 14%.34
The pharmacotherapy management of CKD and any of these comorbid diseases requires multiple medications in addition to dietary restrictions and exercise. As CKD advances to ESRD the pill burden can substantially increase. The daily pill burden for ESRD patients is one of the highest for any chronic disease state. ESRD patients took a mean of 11 ± 4 medications (nine oral and two parenteral) which based on the oral medications resulted in a total pill burden of 19 pills (interquartile range 12) per day. This higher pill burden was associated with a lower quality of life and was not associated with better control of serum phosphorus, a major disease-related problem in ESRD patients.35
Patients receiving renal replacement therapy need to be compliant with not only diet and drugs but also dialysis whether this treatment is daily or three times a week. Compliance is essential to manage these diseases but can seem overwhelming for the patient. Therefore, a good working relationship with healthcare providers including their pharmacist can provide the information and support patients require to actively managing these diseases.
COMPLICATIONS OF HEMODIALYSIS
![]() Complications associated with HD therapy are significant and can limit therapy efficacy. These complications that occur during the actual therapy (intradialytic), as well as those associated with vascular access are discussed in this chapter.36–38
Complications associated with HD therapy are significant and can limit therapy efficacy. These complications that occur during the actual therapy (intradialytic), as well as those associated with vascular access are discussed in this chapter.36–38
Intradialytic Complications
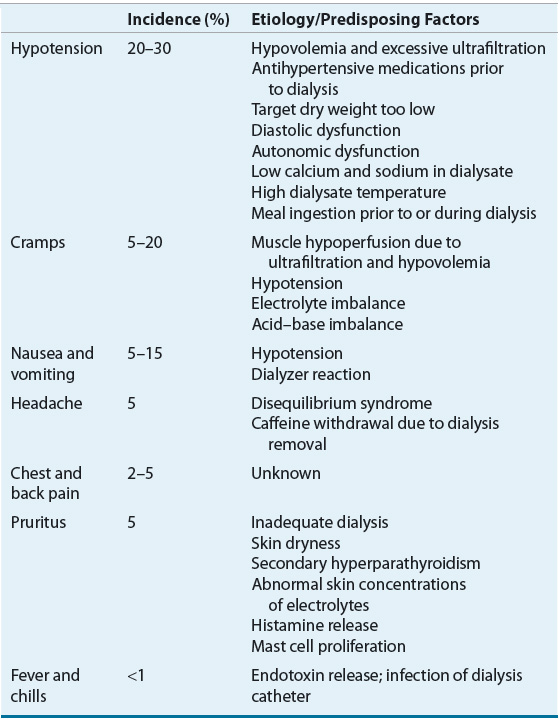
The most common complications that occur during the HD procedure include hypotension, cramps, nausea and vomiting, headache, chest pain, back pain, and fever or chills.37,38 Table 30-4 lists these complications and their etiology and predisposing factors.
A decrease in blood pressure is often noted during HD, but a symptomatic decline in blood pressure that requires nursing or medical intervention can lead to a decrease in the effectiveness of this treatment.37 Intradialytic hypotension (IDH) is primarily related to the rate and amount of fluid removed during32 typical treatments, although other causes, as listed in Table 30-4, may also play a role.39 Other symptoms such as nausea and cramping are often present during acute hypotensive episodes. The replacement of acetate with bicarbonate as the dialysate buffer, the use of volumetric ultrafiltration controllers, as well as individualized dialysate sodium concentrations and modeling have helped reduce the incidence of hypotension.40–42
Skeletal muscle cramps complicate 5% to 20% of HD treatments. Although the pathogenesis of cramps is multifactorial, plasma volume contraction and decreased muscle perfusion caused by excessive ultrafiltration are frequently the initiating events.37,38 Another complication pruritus, which may appear to increase in severity during the HD treatment, is actually a complication of CKD and the management of this condition is discussed in Chapter 29.
Vascular Access Complications
The maintenance of vascular access patency is critical for HD patients since this access is essential for treatment. Aneurysm and stenosis have been reported with AV fistulas and grafts and resolution of this is primarily by surgical means. Thrombosis and infection are the most common vascular access complications with the highest occurrence found in patients with a cuff catheter compared with those with an AV graft or AV fistula.38,43,44
Vascular access dysfunction is usually identified by a decrease in blood flow through the access (blood flow <300 mL/min) over a period of days to weeks. Ultrasound, venography, or computed tomography scans can be used for a definitive diagnosis.22,45,46 Catheter thrombosis can form either inside (intrinsic) or outside (extrinsic) the catheter. The occlusion can form within the lumen at the tip or develop a fibrin sleeve around the catheter where this fibrin sleeve can serve as a nidus for infection and ultimately require catheter removal.47,48
Infection is the second leading cause of mortality in HD patients.49 The risk of sepsis-related death is 100 times greater in dialysis patients than the general population and those with an indwelling catheter have the highest risk.50 Common skin flora such as S. aureus and coagulase-negative staphylococcus are frequently the source of infection, but gram-negative bacterial and fungal causes must not be overlooked. Catheter-related infections develop at the insertion site, hub, or both. The infection source for long-term catheters such as a tunneled catheter is usually the hub where bacteria can enter the blood leading to a bloodstream infection.51,52 Overall HD access with a catheter is associated with higher rates of bacteremia, osteomyelitis, septic arthritis, endocarditis, and death as well as increased treatment costs compared with an AV fistula or AV graft.43
MANAGEMENT OF HEMODIALYSIS COMPLICATIONS
Hypotension
Acute management of IDH includes placing the patient in the Trendelenburg position, decreasing the ultrafiltration rate, lowering the dialysate temperature, modifying dialysate electrolyte concentrations, and/or administering normal or hypertonic saline.36–41 IDH may not occur during each HD session and patient intravariability could necessitate further HD customization. Hypertensive medications administered the day prior to HD therapy may contribute to IDH; therefore, a careful review of all medications including antihypertensive therapies is warranted. Patients with IDH should be counseled to take their blood pressure medications after HD.
IDH is generally related to an insufficient cardiac response to reduced circulating blood volume; therefore, most treatments are directed toward restoring or maintaining adequate blood vessel perfusion in these patients. For example decreasing the dialysate temperature to 36.5°C (97.7°F) may help reduce core body temperature, which can decrease vasodilation.53,54 If nonpharmacologic interventions are not adequate to prevent or reduce the incidence of symptomatic IDH then pharmacologic interventions should be considered (Table 30-5). Several pharmacologic options are discussed in this section.
TABLE 30-5 Management of Hypotension
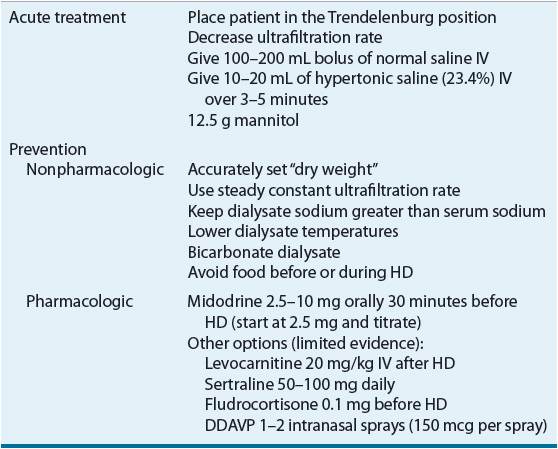
Midodrine, an α1-adrenergic agonist prodrug (active metabolite desglymidodrine), with peripheral vasoconstrictive properties has been effective with managing IDH. Midodrine administered prior to HD in doses ranging from 2.5 to 10 mg resulted in postdialysis blood pressure elevations and improvement of symptoms. An average increase of systolic and diastolic blood pressures was 12.4 and 7.3 mm Hg above the values in control patients, respectively.55 An 8-month long study found that midodrine 10 mg given 30 minutes prior to dialysis resulted in correction of hypotension without any adverse events.56 Oral midodrine (5 mg) given twice daily can increase blood pressure in HD patients with chronic hypotension on nondialysis days.57 It is important to note that the effects of midodrine are probably best in patients with hypotension related to autonomic dysfunction as opposed to other causes of hypotension. The main adverse effect related to midodrine is urinary retention, but patients with peripheral vascular disease should be monitored for digital or lower limb ischemia.58
Other potential therapeutic agents for IDH include levocarnitine, sertraline, and intranasal desmopressin acetate (DDAVP). The IV administration of levocarnitine (20 mg/kg at the end of each dialysis session) reduced hypotensive episodes from 17 to 7 (P <0.02) in a study of 38 patients.59,60 The high cost and limited data on levocarnitine, however, preclude a strong recommendation for its use. Sertraline has demonstrated efficacy in some,61,62 but not all studies.63 A study of 17 IDH patients compared DDAVP to placebo (saline nasal spray).64 Overall, the use of DDAVP increased post-HD blood pressure and decreased the incidence of IDH. In addition, fludrocortisone has been suggested as a potential agent for symptomatic hypotension.65 These medications have limited clinical evidence and should be used with caution in patients with IDH.
Muscle Cramps
Nonpharmacologic interventions related to dialytic therapy may help alleviate muscle cramps. These measures include adjusting the ultrafiltration rate to avoiding hypotension, volume contraction, or hypoosmolality. Other methods to reduce muscle cramps are compression devices, moist heat, massage, exercise, stretching or muscle flexing and should be considered first to minimize adverse consequences (Table 30-6).37,66
TABLE 30-6 Management of Cramps

Both vitamin E and quinine significantly reduce the incidence of muscle cramps.67–69 Quinine is usually well tolerated, but rarely may cause temporary sight and hearing disturbances, thrombocytopenia, or GI distress. Furthermore, quinine tends to increase plasma digoxin concentrations and may enhance the effect of warfarin. This constellation of adverse events prompted the withdrawal of quinine from the over-the-counter market in 1995 and prescription quinine can no longer be marketed for leg cramps.
A randomized, double-blind, placebo-controlled trial demonstrated that both vitamin E (400 mg) and vitamin C (250 mg) reduce the frequency of cramps in dialysis patients.70 The combination of these two drugs had an additive effect. Although these data further strengthen the case for vitamin E, it is unclear what role oral vitamin C would play since many patients are on a renal multiple vitamin containing vitamin C (the current study restricted all vitamin products for 1 month prior to the study). Furthermore, there is some concern that oxalate, a metabolite of vitamin C, may accumulate in dialysis patients and result in systemic oxalosis.
Exogenous administration of creatine might have some beneficial effects on muscle cramps in dialysis patients.71 Ten patients with intradialytic muscle cramps were randomized to either creatine (12 mg before dialysis) or placebo. The frequency of muscle cramps decreased 60% in the creatine group, while there were no differences in the placebo group. Although serum creatinine concentrations rose in the treatment group, no side effects were noted.71 Certainly more research in this area is needed before creatine supplementation can be recommended for the prevention and treatment of muscle cramps during HD.
The relationship of elevated calcium, phosphorus, and intact parathyroid hormone in relation to muscle pain, cramps, pruritus, and dry skin (xerosis) were evaluated in 1,469 HD and PD patients.72 At baseline approximately 67% of patients suffered from at least one of these symptoms. After 4 years of follow-up, those patients with diminished or no symptoms had lower serum phosphorus concentrations.72 This study suggests that adequate dialysis along with dietary controls can help reduce muscle pain, cramps, itching, and dry skin in dialysis patients.
Shakuyaku-kanzo-to, a combination of peony and licorice root from traditional Japanese and Chinese medicine, was studied in 23 HD patients for acute treatment of muscle cramps. The ultrafiltration rate was reduced to zero and shakuyaku-kanzo-to (2.5 gram granule) was administered when a patient complained of cramping during HD. These interventions resulted in a muscle cramp resolution rate of 88.5% that occurred between 5 and 10 minutes.73 It is difficult to determine if muscle cramp cessation was due to stopping HD fluid removal, shakuyaku-kanzo-to, or the combination of both treatments. Previous studies have examined the use45 of shakuyaku-kanzo-to for the frequency and severity of muscle cramps but the results were inconsistent and a few patients had an increase in symptoms.74
Pharmacologic interventions to diminish muscle cramps are limited and currently vitamin E has the strongest evidence-based clinical trials and its safety profile. Quinine sulfate is available as Qualaquin 324 mg capsule (URL Pharma, Philadelphia, PA) but is FDA approved only for malaria. The FDA has warned against the off-label use of quinine for muscle cramps because of potential serious side effects related to its use. The dosage for HD-related muscle cramps is one capsule (324 mg) either at bedtime or 1 to 2 hours prior to HD.
Vascular Access Thrombosis
Prevention of vascular access thrombus formation is a key component to maintain this lifeline for HD patients. Multiple oral and IV anticoagulant and antiplatelet agents and IV thrombolytic agents have been studied for vascular access patency. Several of these therapeutic options are discussed in this section.