Chapter 11 Heart Sounds and Extra Sounds
B. Cardiac Auscultation: Some Suggestions
1 Why is cardiac auscultation so difficult?
 Cardiac sounds are often at the threshold of audibility. The human ear can only perceive sounds between 20 and 20,000 Hz: it can neither reach beyond (as dolphins and whales do) nor go below it (as elephants often do). Yet, in this range, it has a preferential bandwidth of 1000–5000 Hz, corresponding to that of the human voice. Yet, most cardiac sounds are <500 Hz. In fact, many are so low pitched to be almost inaudible (S3 or S4 can be <100 Hz).
Cardiac sounds are often at the threshold of audibility. The human ear can only perceive sounds between 20 and 20,000 Hz: it can neither reach beyond (as dolphins and whales do) nor go below it (as elephants often do). Yet, in this range, it has a preferential bandwidth of 1000–5000 Hz, corresponding to that of the human voice. Yet, most cardiac sounds are <500 Hz. In fact, many are so low pitched to be almost inaudible (S3 or S4 can be <100 Hz).
 Cardiac sounds are crammed in a very little time interval. At a rate of 70/minute, a cycle of 0.8 seconds can easily harbor four to five sounds, many barely detectable.
Cardiac sounds are crammed in a very little time interval. At a rate of 70/minute, a cycle of 0.8 seconds can easily harbor four to five sounds, many barely detectable.
 Patients’ hair and respiration create misleading artifacts.
Patients’ hair and respiration create misleading artifacts.
 The obesity epidemic has given many patients a much fattier chest muffler.
The obesity epidemic has given many patients a much fattier chest muffler.
 Pathology has shifted from rheumatic to coronary, thus reducing the pool of teaching patients.
Pathology has shifted from rheumatic to coronary, thus reducing the pool of teaching patients.
 Our ever-increasing fascination with the inanimate and the machine (and the sophistication of technology), compounded by:
Our ever-increasing fascination with the inanimate and the machine (and the sophistication of technology), compounded by:
2 How can you make auscultation a little easier?
 Separate systole from diastole (easily done in normal rates by recognizing the acoustic differences of S1 and S2 plus the long and short intervals; in faster hearts, it may require simultaneous assessment of the arterial pulse or precordial impulse).
Separate systole from diastole (easily done in normal rates by recognizing the acoustic differences of S1 and S2 plus the long and short intervals; in faster hearts, it may require simultaneous assessment of the arterial pulse or precordial impulse).
 “Inch” (move your stethoscope inch by inch, from auscultatory area to auscultatory area).
“Inch” (move your stethoscope inch by inch, from auscultatory area to auscultatory area).
 Know how to use your tool: (1) bell versus diaphragm; (2) patient’s position (supine, seated, and left lateral decubitus); (3) changes with respiration; and (4) dynamic bedside maneuvers (straight-leg raising, squatting-standing, Valsalva, hand-gripping, exercise).
Know how to use your tool: (1) bell versus diaphragm; (2) patient’s position (supine, seated, and left lateral decubitus); (3) changes with respiration; and (4) dynamic bedside maneuvers (straight-leg raising, squatting-standing, Valsalva, hand-gripping, exercise).
 When challenged by feeble and crammed signals, focus on one sound at a time.
When challenged by feeble and crammed signals, focus on one sound at a time.
 Develop pattern recognition. This means practice, practice, practice…. In fact, you may need to hear an individual acoustic event as many as 500 times before you can master it.
Develop pattern recognition. This means practice, practice, practice…. In fact, you may need to hear an individual acoustic event as many as 500 times before you can master it.
C. Normal Heart Sounds
5 What are the cardiac areas?
They are areas of chest wall projection that correspond to the four cardiac valves (see Chapter 12, questions 1 and 2). In a clockwise fashion:
 Aortic area: Second right parasternal interspace
Aortic area: Second right parasternal interspace
 Pulmonic area: Second left parasternal interspace
Pulmonic area: Second left parasternal interspace
 Erb’s point: Third left parasternal interspace (area of left ventricular outflow)
Erb’s point: Third left parasternal interspace (area of left ventricular outflow)
 Mitral area: Apex (fifth interspace left midclavicular line)
Mitral area: Apex (fifth interspace left midclavicular line)
 Tricuspid area: Fourth to fifth left parasternal space, at times extending into the epigastrium/subxiphoid
Tricuspid area: Fourth to fifth left parasternal space, at times extending into the epigastrium/subxiphoid
(1) First Heart Sound (S1)
6 Where is S1 best heard?
At the apex (for its mitral component) and over the subxiphoid/epigastrium (for the tricuspid).
7 How is S1 generated?
By the vibration of valves, ventricles, and blood that coincides with:
1. Closure of the atrioventricular (A-V) valves
2. Opening of the semilunar valves. This in turn leads to two separate sounds, caused by:
In the absence of pathology, only A-V closure is responsible for S1. Semilunar opening is silent.
8 Which characteristics of S1 are clinically valuable and should therefore be identified?
The most valuable is intensity (and variations thereof). The next most valuable is splitting.
10 What is the significance of S2 being louder than S1 at the apex?
It suggests two possible explanations:
11 Which factors are responsible for the loudness of S1?
In addition to shape and thickness of the chest wall, three major factors play a role:
1. The rate of rise in left ventricular pressure: This is a function of ventricular contractility, with stronger contractions causing a faster rise in left ventricular pressure and thus brisker and more forceful A-V closure. Hence, a loud S1 is typical of the hyperkinetic heart syndrome, whereas a soft (muffled) S1 is instead common in congestive heart failure, whose failing ventricles can only generate a slow rise in systolic pressure.
2. The separation between atrioventricular leaflets at the onset of ventricular systole: The closer the leaflets, the softer S1 is; conversely, the wider apart the leaflets, the louder S1 is. This mechanism feeds into two other important variables:
 The duration of the P-R interval: A short P-R forces the ventricles to contract while the leaflets are still widely separated, so that their closure occurs on a steeper part of the left ventricular pressure curve. This, in turn, means a more forceful and louder closure. Conversely, a long P-R provides enough time for the leaflets to come close to each other, thus softening S1. A muffled S1 used to be quite common in rheumatic fever with first- degree A-V block. The progressive P-R lengthening of the Wenckebach phenomenon may also gradually (and increasingly) soften S1.
The duration of the P-R interval: A short P-R forces the ventricles to contract while the leaflets are still widely separated, so that their closure occurs on a steeper part of the left ventricular pressure curve. This, in turn, means a more forceful and louder closure. Conversely, a long P-R provides enough time for the leaflets to come close to each other, thus softening S1. A muffled S1 used to be quite common in rheumatic fever with first- degree A-V block. The progressive P-R lengthening of the Wenckebach phenomenon may also gradually (and increasingly) soften S1. The atrioventricular pressure gradient: A large A-V pressure gradient keeps the leaflets widely separated until ventricular pressure rises high enough to shut them close. Since the closure takes place on a steeper part of the left ventricular pressure curve, it will be forceful and loud. Hence, the longer the ventricle has to contract in order to close the A-V valve, the louder S1 will be. This is quite common in mitral stenosis, where it contributes to the loudness of S1.
The atrioventricular pressure gradient: A large A-V pressure gradient keeps the leaflets widely separated until ventricular pressure rises high enough to shut them close. Since the closure takes place on a steeper part of the left ventricular pressure curve, it will be forceful and loud. Hence, the longer the ventricle has to contract in order to close the A-V valve, the louder S1 will be. This is quite common in mitral stenosis, where it contributes to the loudness of S1.3. The thickness of the atrioventricular leaflet: The thicker the leaflets, the louder S1 is (banging hardbacks against each other generates more noise than banging paperbacks). Still, a soft S1 may indicate leaflets that are too rigid. Hence, a thickened and stenotic mitral valve may generate a booming S1 early on in the disease, but a softer (or absent) S1 when the leaflets get eventually calcified and fixed.
13 Which diseases present with a variable intensity of S1?
Heart blocks, such as second degree (i.e., Mobitz I or Wenckebach) and third degree:
 In second-degree A-V block, there is progressive softening of S1, while S2 remains constant. This is due to the increasing P-R lengthening, until a beat is eventually dropped. It is so typical of Mobitz I that Wenckebach could describe it even before electrocardiogram (ECG) availability.
In second-degree A-V block, there is progressive softening of S1, while S2 remains constant. This is due to the increasing P-R lengthening, until a beat is eventually dropped. It is so typical of Mobitz I that Wenckebach could describe it even before electrocardiogram (ECG) availability.
 In third-degree A-V block (typical of Morgagni-Adams-Stokes syndrome), the change in S1 intensity is instead random and chaotic because the atrium and ventricle march to the beat of a different drummer, with rates that are totally independent—when ventricular contraction catches the A-V valves wide apart, S1 booms; when it catches them partially closed, S1 softens. The varying S1 intensity is so typically random to allow the recognition of complete block just on the basis of auscultation (Table 11-1).
In third-degree A-V block (typical of Morgagni-Adams-Stokes syndrome), the change in S1 intensity is instead random and chaotic because the atrium and ventricle march to the beat of a different drummer, with rates that are totally independent—when ventricular contraction catches the A-V valves wide apart, S1 booms; when it catches them partially closed, S1 softens. The varying S1 intensity is so typically random to allow the recognition of complete block just on the basis of auscultation (Table 11-1).
| Loud | Variable | Soft |
|---|---|---|
| Short P-R interval (<160 msec) | Atrial fibrillation | Long P-R interval (>200 msec) |
| Increased contractility (hyperkinetic states) | Atrioventricular block (Wenckebach and third degree) | Decreased contractility (left ventricular dysfunction) |
| Thickening of mitral (or tricuspid) leaflets | Ventricular tachycardia (due to atrioventricular dissociation) | Left bundle branch block |
| Increased atrioventricular pressure gradient (stenosis of the A-V valves) | Pulsus alternans | Calcification of A-V valve(s) |
| Premature closure of mitral valve (acute aortic regurgitation) | ||
| Mitral (or tricuspid) regurgitation |
18 How is S1 in mitral stenosis (MS)?
 Thickening of the mitral leaflets: In the late stages of MS, however, leaflets can become stiff and poorly mobile, which, in turn, softens S1 and eventually eliminates it.
Thickening of the mitral leaflets: In the late stages of MS, however, leaflets can become stiff and poorly mobile, which, in turn, softens S1 and eventually eliminates it.
 High atrioventricular pressure gradient: This is produced by the stenotic valve and keeps the A-V leaflets maximally separated at the onset of ventricular contraction.
High atrioventricular pressure gradient: This is produced by the stenotic valve and keeps the A-V leaflets maximally separated at the onset of ventricular contraction.
19 What other conditions can be associated with a loud S1?
 Holosystolic mitral valve prolapse with regurgitation (where the prolapse delays the tension of the redundant mitral leaflet, thus allowing it to occur at peak of ventricular contraction, which makes it louder). A similar mechanism takes place in:
Holosystolic mitral valve prolapse with regurgitation (where the prolapse delays the tension of the redundant mitral leaflet, thus allowing it to occur at peak of ventricular contraction, which makes it louder). A similar mechanism takes place in:
 A left-atrial myxoma. Here it is the tumor that delays the closure of the mitral valve, thus allowing it to occur at peak of ventricular contraction and making it, therefore, louder. As a result, 80% of patients with this condition will have a loud S1.
A left-atrial myxoma. Here it is the tumor that delays the closure of the mitral valve, thus allowing it to occur at peak of ventricular contraction and making it, therefore, louder. As a result, 80% of patients with this condition will have a loud S1.
 Short P-R interval, as in the pre-excitation syndromes of Wolff-Parkinson-White and Ganong-Levine syndromes.
Short P-R interval, as in the pre-excitation syndromes of Wolff-Parkinson-White and Ganong-Levine syndromes.
(2) Second Heart Sound (S2)
31 How is S2 generated?
By sudden deceleration of blood following the closure of aortic (A2) and pulmonic (P2) valves.
32 Which of the two semilunar valve closes earlier?
The aortic, due to systemic pressure being normally higher than pulmonic pressure.
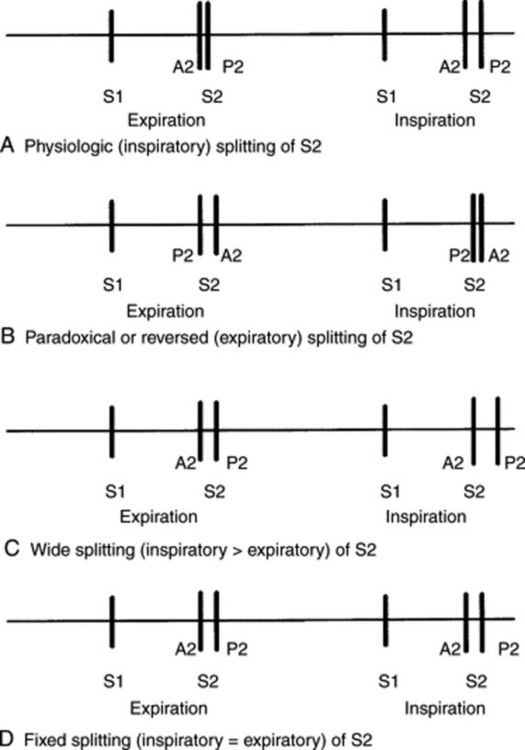
35 What is a physiologic splitting of S2?
It is the inspiratory widening of the normal interval between A2 and P2. This is triggered by:
 Increased venous return to the right ventricle (due to negative intrathoracic pressure). This delays P2.
Increased venous return to the right ventricle (due to negative intrathoracic pressure). This delays P2.
 Decreased venous return to the left ventricle (due to pooling of blood in the lungs). This anticipates A2.
Decreased venous return to the left ventricle (due to pooling of blood in the lungs). This anticipates A2.
37 How common is a physiologic splitting of S2?
Not very common. In a study of 196 normal adults examined in the supine position, only 52.1% had an audible inspiratory split of S2. Physiologic splitting was much more common in younger individuals (60% of those between ages 21 to 30, and 34% of those older than 50). Indeed, after age 50, S2 appeared single in more than 60% of subjects, as opposed to 36% for all ages. Hence, in older patients a single S2 should not be considered evidence for a delayed A2 (and therefore it should not suggest underlying aortic stenosis [AS] or a left bundle branch block) (see Fig. 11-1).
38 Why does S2 splitting disappear with aging?
Because of senile emphysema, with greater air muffling of the pulmonic component of S2.
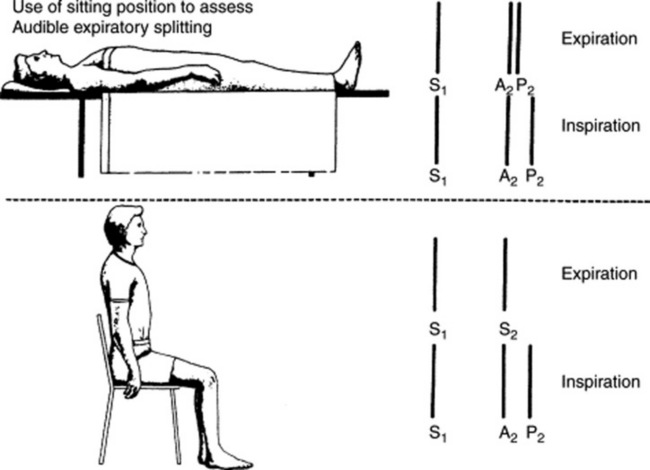
39 How important is a patient’s position on S2 splitting?
Very important. A supine position increases venous return, lengthens right ventricular systole, and thus widens the physiologic splitting of S2. Conversely, a sitting (or standing) position decreases venous return, shortens right ventricular systole, and narrows the physiologic split (Fig. 11-2). This is especially important when analyzing an expiratory splitting of S2. In a study by Adolph and Fowler, 22/200 (11%) normal subjects had an expiratory split while supine, but only 1/22 maintained it upon sitting or standing. Hence, a true expiratory splitting of S2 is one that is present both in a recumbent and upright position.
40 What is the significance of a true expiratory splitting of S2?
It indicates one of three conditions:
44 What is a fixed splitting of S2? What does it mean?
It is an S2 that remains audibly split throughout respiration, both in the supine and upright positions, and with a consistent interval between its two components. Although encountered in severe ventricular failure, a fixed splitting of S2 should suggest a septal defect (most often atrial but occasionally ventricular), especially if associated with pulmonary hypertension. The defect (and its shunt) eliminate the respiratory changes in right and left ventricular stroke volume, thus fixing the S2 splitting (more rarely, a fixed S2 split will occur in severe impedance to right ventricular emptying, such as that of pulmonary stenosis, pulmonary hypertension, or massive pulmonary embolism—with or without bundle branch block). These patients cannot cope with the increased venous return of inspiration by increasing right ventricular stroke volume. Hence, they maintain their S2 widely and persistently split throughout respiration (see Fig. 11-3).
45 What is the differential diagnosis of a fixed splitting of S2?
A late-systolic click (which precedes S2) and an early diastolic extra sound (which follows S2):
 The late-systolic click varies with bedside maneuvers and is loudest at the apex (conversely, the split S2 is unchanged with maneuvers and only heard at the base).
The late-systolic click varies with bedside maneuvers and is loudest at the apex (conversely, the split S2 is unchanged with maneuvers and only heard at the base).
 The two most common early diastolic extra sounds are the S3 and the opening snap (OS) of mitral (or tricuspid) stenosis (for a discussion of how to differentiate an opening snap from a widely split S2 or an S3, see questions 103, 104, and 130). OS is primarily apical, whereas the split S2 is basilar. Still, OS can be loud enough to transmit to the base, thus producing a triple lilt in inspiration (OS + split S2, with a loud P2 because of pulmonary hypertension). Note that the interval between S2 and OS is wider than that between the two components of S2. Finally, an OS is usually (but not necessarily) associated with a diastolic rumble.
The two most common early diastolic extra sounds are the S3 and the opening snap (OS) of mitral (or tricuspid) stenosis (for a discussion of how to differentiate an opening snap from a widely split S2 or an S3, see questions 103, 104, and 130). OS is primarily apical, whereas the split S2 is basilar. Still, OS can be loud enough to transmit to the base, thus producing a triple lilt in inspiration (OS + split S2, with a loud P2 because of pulmonary hypertension). Note that the interval between S2 and OS is wider than that between the two components of S2. Finally, an OS is usually (but not necessarily) associated with a diastolic rumble.
48 What are the causes of paradoxical S2 splitting?
 Delayed aortic closure. This is indeed the most common reason, usually due to a complete left bundle branch block (where reversed S2 splitting can occur in 84% of the cases). Other causes include increased impedance to left ventricular emptying (hypertension, AS, coarctation) or left ventricular dysfunction. The latter can occur in acute ischemia and various cardiomyopathies.
Delayed aortic closure. This is indeed the most common reason, usually due to a complete left bundle branch block (where reversed S2 splitting can occur in 84% of the cases). Other causes include increased impedance to left ventricular emptying (hypertension, AS, coarctation) or left ventricular dysfunction. The latter can occur in acute ischemia and various cardiomyopathies.
 Early pulmonic closure. This is a much less common cause of paradoxical splitting, usually due to decreased right ventricular filling—from either tricuspid regurgitation or right atrial myxoma.
Early pulmonic closure. This is a much less common cause of paradoxical splitting, usually due to decreased right ventricular filling—from either tricuspid regurgitation or right atrial myxoma.
50 What is the significance of a “single splitting” of S2?
 Aging: The audible splitting of S2 decreases in prevalence with age, to the point of becoming absent in most subjects older than 60. This is probably due to the muffling of P2 by the “physiologic” senile emphysema.
Aging: The audible splitting of S2 decreases in prevalence with age, to the point of becoming absent in most subjects older than 60. This is probably due to the muffling of P2 by the “physiologic” senile emphysema.
 Emphysema: The hyperinflated lungs will muffle P2 during inspiration, thus making A2 the only audible sound. Because this phenomenon is less pronounced in exhalation, these patients may be misdiagnosed as having paradoxical splitting of S2 (while, in fact, they have a pseudo-paradoxical splitting that becomes evident only in expiration).
Emphysema: The hyperinflated lungs will muffle P2 during inspiration, thus making A2 the only audible sound. Because this phenomenon is less pronounced in exhalation, these patients may be misdiagnosed as having paradoxical splitting of S2 (while, in fact, they have a pseudo-paradoxical splitting that becomes evident only in expiration).
 Reversed (or paradoxical) splitting: In this case, the split will indeed occur only in exhalation.
Reversed (or paradoxical) splitting: In this case, the split will indeed occur only in exhalation.
 Pulmonary hypertension: Increased impedance on right ventricular emptying makes the ventricle unable to cope with the increased venous return of inspiration. Hence, there will be no inspiratory lengthening of right ventricular systole and no inspiratory splitting of S2.
Pulmonary hypertension: Increased impedance on right ventricular emptying makes the ventricle unable to cope with the increased venous return of inspiration. Hence, there will be no inspiratory lengthening of right ventricular systole and no inspiratory splitting of S2.
 Semilunar valvular disease: Stiffening and reduced mobility of semilunar valves may also lead to the disappearance of either A2 or P2, thus making S2 “single.”
Semilunar valvular disease: Stiffening and reduced mobility of semilunar valves may also lead to the disappearance of either A2 or P2, thus making S2 “single.”
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree