Figure 13-1. Acute Decompensated Heart Failure
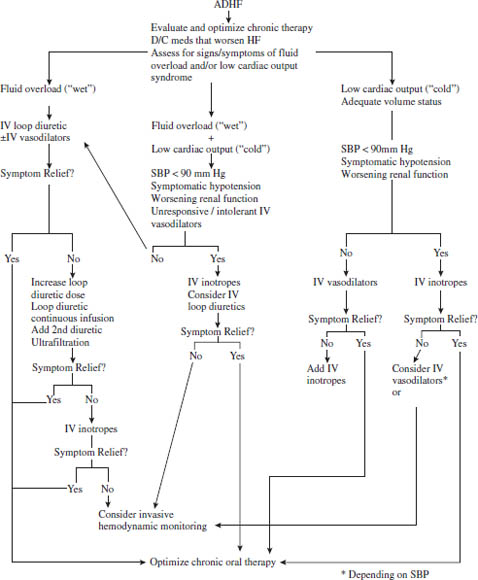
Parker RB, Rodgers JE, Cavallari LH, 2008.
SBP, systolic blood pressure; D/C, discontinue.
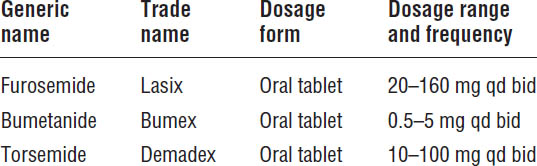
Loop Diuretics
Only patients with signs or symptoms of volume overload will need diuretic therapy. Most heart failure patients require use of the more potent loop diuretics instead of thiazide diuretics (Table 13-1).
Mechanism of action
Loop diuretics reduce the sodium and fluid retention associated with heart failure by inhibiting reabsorption of sodium and chloride in the loop of Henle.
Patient instructions and counseling
■ Patients allergic to sulfa-containing medications also may be allergic to loop diuretics.
■ Patients should take medication once a day in the morning or, if taking twice daily, in the morning and afternoon.
■ Loop diuretics can cause frequent urination.
■ Patients should weigh themselves daily (preferably in the morning, after urinating). Patients who gain more than 1 pound per day for several consecutive days or 3–5 pounds in a week should contact their health care provider.
■ Patients should report muscle cramps, dizziness, excessive thirst, weakness, or confusion, as these may be signs of overdiuresis.
■ Patients should avoid sun exposure or use sunscreen when taking loop diuretics.
Adverse drug events
■ Electrolyte depletion: hypokalemia and hypomagnesemia
■ Hypotension
■ Renal insufficiency
Drug–drug and drug–disease interactions
■ Food decreases the bioavailability of furosemide and bumetanide, so these agents should be taken on an empty stomach. Food does not affect torsemide absorption.
■ The absorption of oral furosemide is slowed significantly in patients with ADHF, resulting in decreased diuretic response. Therefore, those individuals usually will require the use of intravenous (IV) furosemide.
■ NSAIDs may diminish these agents’ diuretic effects.
■ Potassium supplementation may not be required in patients also receiving ACEIs, ARBs, or aldosterone antagonists.
Parameters to monitor
■ Serum sodium, potassium, magnesium, creatinine, and blood urea nitrogen (BUN)
■ Patient weight (a loss of 0.5–1 kg daily is desired until the patient achieves the desired dry weight)
■ Urine output
■ Blood pressure
■ Jugular venous distension
■ Improvement in heart failure symptoms (dyspnea and peripheral edema)
Kinetics
The bioavailability of torsemide is not affected by food and is less variable than that of furosemide.
Angiotensin-Converting Enzyme Inhibitors
ACEIs are recommended for all patients with HFrEF and current or prior symptoms of heart failure, unless contraindicated (Table 13-2). Clinical trials with more than 7,000 patients consistently demonstrate that ACEIs alleviate symptoms, improve clinical status and quality of life, and reduce mortality.
Mechanism of action
■ ACEIs interfere with the renin–angiotensin system by inhibiting the angiotensin-converting enzyme, which is responsible for the conversion of angiotensin I to the potent vasoconstrictor angiotensin II. This inhibition results in a decrease in plasma angiotensin II and aldosterone concentrations, thus reducing the adverse effects of those neurohormones. Inhibition of the angiotensin-converting enzyme also prevents the breakdown of the endogenous vasodilator bradykinin.
■ ACEIs reduce heart failure symptoms and decrease hospitalizations for heart failure.
■ ACEIs reduce mortality by 20–30% and slow the progression of heart failure.
Patient instructions and counseling
■ Patients who are pregnant or breast-feeding should not take ACEIs. If patients become pregnant while taking an ACEI, they should contact their physician immediately.
Table 13-2. Angiotensin-Converting Enzyme Inhibitors
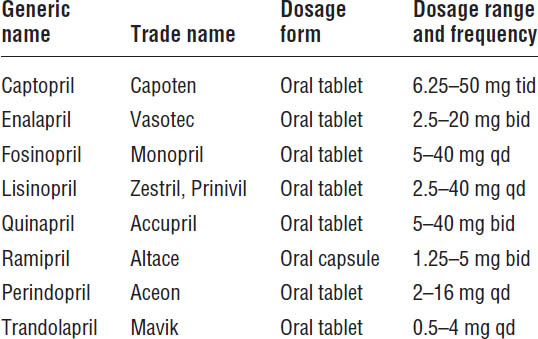
■ Captopril should be taken on an empty stomach, either 1 hour before or 2 hours after meals.
■ Salt substitutes that contain potassium should be used cautiously.
■ Patients should call their physician immediately if they experience swelling of the face, eyes, lips, tongue, arms, or legs or if they have difficulty breathing or swallowing.
■ ACEIs may cause a cough.
Adverse drug events
■ Hypotension
■ Dizziness
■ Renal insufficiency
■ Cough
■ Angioedema
■ Hyperkalemia
■ Rash
■ Taste disturbances
Drug–drug and drug–disease interactions
■ NSAIDs can increase the risk of renal insufficiency and attenuate the beneficial effects of ACEIs.
■ Potassium supplements or potassium-sparing diuretics should be used with caution.
■ Cyclosporine and tacrolimus may increase the risk of nephrotoxicity and hyperkalemia.
■ Diuretics increase the risk of hypotension.
Parameters to monitor
■ Blood pressure
■ Renal function (i.e., serum BUN and creatinine)
■ Serum potassium
■ Heart failure symptoms
■ Dose (initiate therapy at low doses; if lower doses are tolerated well, follow with gradual increases)
Other
ACEIs are pregnancy category C during the first trimester and pregnancy category D during the second and third trimesters. ACEIs can cause fetal and neonatal morbidity and death when administered to pregnant women.
Angiotensin II Receptor Blockers
ACEIs remain the drugs of choice for inhibiting the renin–angiotensin–aldosterone system in patients with HFrEF. Recent clinical trials confirm the efficacy and safety of candesartan and valsartan in this population. Current guidelines recommend candesartan or valsartan for patients who are intolerant to ACEIs—both agents are approved for use in patients with heart failure. Intolerance to ACEIs is most often due to cough or angioedema, although caution is advised when using ARBs in patients who have angioedema secondary to an ACEI. Note that ARBs are just as likely as ACEIs to cause impaired renal function, hyperkalemia, or hypotension (Table 13-3).
Table 13-3. Angiotensin II Receptor Blockers
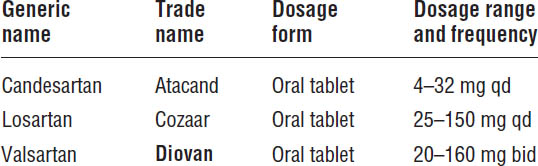
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
Mechanism of action
■ ARBs interfere with the renin–angiotensin system by blocking the angiotensin-1 receptor, thereby attenuating the detrimental effects of this hormone.
■ Unlike ACEIs, ARBs do not affect the kinin system and thus are not associated with cough.
■ ARBs reduce hospitalizations and improve survival.
Patient instructions and counseling
■ Patients who are pregnant or breast-feeding should not take ARBs. If a patient becomes pregnant while taking an ARB, she should contact her physician immediately.
■ Use salt substitutes that contain potassium cautiously.
■ Dizziness or light-headedness may occur, especially in patients taking diuretics.
Adverse drug events
■ Hypotension
■ Dizziness
■ Renal insufficiency
■ Hyperkalemia
Drug–drug and drug–disease interactions
■ Potassium supplements or potassium-sparing diuretics should be used with caution.
■ Diuretics increase the risk of hypotension.
Parameters to monitor
■ Blood pressure
■ Renal function (i.e., serum BUN and creatinine)
■ Serum potassium
■ Heart failure symptoms
■ Dose (initiate therapy at low doses; if lower doses are tolerated well, follow with gradual increases)
Other
■ ARBs are pregnancy category C during the first trimester and pregnancy category D during the second and third trimesters. ARBs can cause fetal and neonatal morbidity and death when administered to pregnant women.
β-Blockers
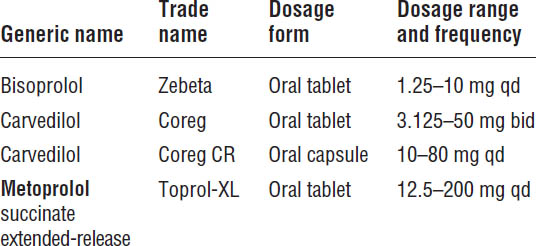
Because of their negative inotropic effects, β-blockers were once considered to be contraindicated in patients with HFrEF. However, by inhibiting the deleterious effects of long-term activation of the sympathetic nervous system in heart failure, β-blockers repeatedly have been shown to provide hemodynamic, symptomatic, and survival benefits. Metoprolol succinate (extended-release metoprolol), bisoprolol, and carvedilol all have been shown to be effective, and one of these three agents should be used for the treatment of HFrEF (Table 13-4).
Mechanism of action
■ Blockade of β-receptors antagonizes the increase in sympathetic nervous system activity, which is one of the significant mechanisms responsible for the progression of heart failure. Bisoprolol and metoprolol succinate are β1-selective agents, whereas carvedilol blocks β1-, β2-, and α1-receptors. Whether these differences in pharmacologic actions have any important effects on outcomes remains uncertain.
Boldface indicates one of top 100 drugs for 2012 by units sold at retail outlets, www.drugs.com/stats/top100/2012/units.
■ Treatment with β-blockers reduces symptoms, improves clinical status, and decreases the risk of death and hospitalization.
■ One of the three β-blockers that have been shown to reduce mortality (bisoprolol, carvedilol, and extended-release metoprolol succinate) should be used in all stable patients with HFrEF and current or prior heart failure symptoms, unless contraindicated.
■ In general, β-blockers should be used in combination with ACEIs or ARBs and diuretics.
Patient instructions and counseling
■ β-blockers may cause fluid retention or worsening of heart failure upon initiation of therapy or after an increase in dose. Patients should report any cases of body or leg swelling or increased shortness of breath. Patients should weigh themselves daily; if they gain more than 1 pound per day for several consecutive days or 3–5 pounds in a week, they should contact their health care provider.
■ Fatigue or weakness may occur in the first few weeks of treatment but usually will resolve spontaneously.
■ Patients should report any cases of dizziness, lightheadedness, or blurred vision, which may be caused by the patient’s blood pressure being too low or from bradycardia or heart block.
■ Patients should take carvedilol with food.
■ It is important not to miss doses or stop taking these medications abruptly.
■ In patients with diabetes, β-blockers can increase blood sugar and may also mask the signs of hypoglycemia (except for sweating).
Adverse drug events
A list of adverse events most commonly observed in heart failure patients receiving β-blockers follows. For other adverse effects of β-blockers, see Chapter 12 on hypertension and Chapter 15 on ischemic heart disease.
■ Fluid retention and worsening heart failure
■ Fatigue
■ Bradycardia and heart block
■ Hypotension
■ Abrupt withdrawal can lead to hypertension, tachycardia, or myocardial ischemia
Drug–drug and drug–disease interactions
■ Amiodarone and calcium channel blockers (verapamil and diltiazem) can increase the risk of bradycardia, heart block, and hypotension.
■ Quinidine, fluoxetine, paroxetine, and other inhibitors of cytochrome P4502D6 inhibit hepatic metabolism of metoprolol and carvedilol and may result in increased plasma concentrations and enhanced effects.
■ Concomitant use of ophthalmic β-blockers may increase the risk of bradycardia, heart block, and hypotension.
■ β-blockers may cause bronchoconstriction in patients with asthma or COPD.
■ Do not use β-blockers in patients with symptomatic bradycardia or heart block unless a pacemaker is present.
■ β-blockers may worsen blood glucose control in diabetics and mask the signs of hypoglycemia.
Parameters to monitor
■ Blood pressure and heart rate
■ Heart failure symptoms
■ Weight (daily)
Kinetics
■ Bisoprolol is eliminated about 50% by the kidneys, so dosage adjustment may be required in patients with renal insufficiency.
■ Both metoprolol and carvedilol are metabolized by the liver.
Other
■ Patients should be stable (i.e., minimal evidence of fluid overload or volume retention) before β-blocker treatment is initiated.
■ Treatment should be initiated with low doses and titrated slowly upward until the target dose is reached. Doses usually are increased no more frequently than every 2 weeks, with close monitoring of symptoms required during the titration period.
■ Fluid accumulation during dose titration usually can be managed by adjusting diuretic doses.
■ Staggering the schedule of other heart failure medications that lower blood pressure (e.g., ACEIs and diuretics) may help reduce the risk of hypotension.
■ A recent study comparing the effects of carvedilol with immediate-release metoprolol (metoprolol tartrate) in patients with heart failure found that survival is improved in patients receiving carvedilol. Whether carvedilol is superior to extended-release metoprolol (metoprolol succinate) is unknown. However, these results strongly suggest that only β-blockers proven to improve survival (carvedilol, metoprolol succinate, and bisoprolol) should be used in patients with HFrEF as recommended by current guidelines.
Aldosterone Receptor Antagonists
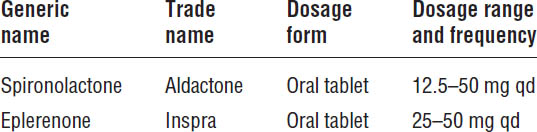
Elevated plasma aldosterone plays an important detrimental role in the pathophysiology and progression of heart failure. Although short-term treatment with ACEIs or ARBs lowers circulating aldosterone concentrations, this suppression is not sustained with long-term therapy. In low doses, the aldosterone antagonists—spironolactone and eplerenone—reduce the risk of death and hospitalization in patients with moderate to severe heart failure. Current guidelines recommend the addition of aldosterone antagonists in patients with NYHA class II–IV HFrEF who can be monitored closely for renal function and serum potassium (Table 13-5). Aldosterone antagonists are also recommended for patients with acute myocardial infarction and an LVEF ≤ 40%, as well as heart failure symptoms or a history of diabetes.
Mechanism of action
Aldosterone plays an important role in heart failure pathophysiology. In addition to increasing renal sodium retention and potassium loss, aldosterone is also a key mediator of ventricular hypertrophy and remodeling, which drives the initiation and progression of heart failure. Antagonism of aldosterone receptors by spironolactone and eplerenone attenuates these detrimental effects.
Patient instructions and counseling
■ Potassium-containing salt substitutes should be avoided.
Table 13-5. Aldosterone Antagonists
■ Patients should call their physician immediately if they experience muscle weakness or cramps; numbness or tingling in hands, feet, or lips; or slow or irregular heartbeat.
■ Spironolactone may cause swollen or painful breasts in men.
Adverse drug events
■ Hyperkalemia
■ Gynecomastia (only with spironolactone)
■ Irregular menses
Drug–drug and drug–disease interactions
■ ACEIs, ARBs, and NSAIDs increase the risk of hyperkalemia.
■ Spironolactone can increase digoxin plasma concentrations.
■ Potassium supplements increase the risk of hyperkalemia. Supplements should not be used if serum potassium > 3.5 mEq/L.
■ Elderly patients and patients with diabetes are at an increased risk of hyperkalemia.
■ Erythromycin, clarithromycin, verapamil, ketoconazole, fluconazole, itraconazole, and other inhibitors of cytochrome P4503A4 inhibit hepatic metabolism of eplerenone and may result in increased plasma concentrations and enhanced effects.
Parameters to monitor
■ Serum creatinine should be < 2.5 mg/dL in men or < 2 mg/dL in women (or estimated glomerular filtration rate > 30 ml/min for men and women) before therapy is initiated.
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree


