Fig. 18.1
Major drugs of abuse targets for immunopharmacotherapy
18.1 Drug Target Consideration for Hapten Design
Many of the principles of hapten design are generally applicable to any target drug vaccine: adhere as close to the structure of the natural drug molecule as possible, attach the linker (used to conjugate to the carrier protein) such that appropriate epitopes are displayed for recognition by the immune system, and attempt to minimize any inherent linker-derived immunogenicity. However, other design factors require consideration of the structure and pharmacology of the target drug, including metabolic or hydrolytic instability, the requirement for target selectivity, and the ease of hapten synthesis. In this section, the choice of hapten for each of the five main targets for drugs of abuse vaccines is discussed in the context of the specific challenges faced in obtaining the desired immunological response (Fig. 18.2).
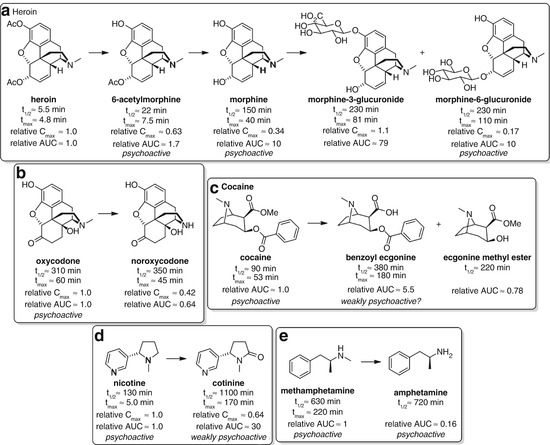

Fig. 18.2
Major metabolites of heroin (Skopp et al. 1997; Osborne et al. 1992; Rook et al. 2006a, b), oxycodone (Pöyhiä et al. 1992), cocaine (Jeffcoat et al. 1989; Jufer et al. 2000; Kolbrich et al. 2006), nicotine (Hukkanen et al. 2005; Yuki et al. 2013) and methamphetamine (Schep et al. 2010; Barceloux 2012) in humans
18.1.1 Heroin
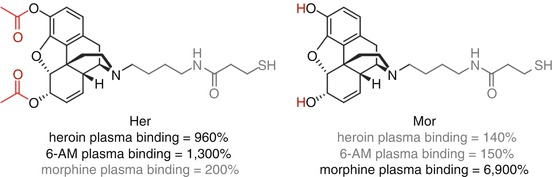
Heroin provides a complex example that highlights the wide range of challenges involved in hapten design. The acetyl groups of heroin are rapidly hydrolyzed in vivo to give 6-acetylmorphine (6-AM) and subsequently morphine, before glucuronidation to give morphine-6-glucuronide or its 3-isomer (Fig. 18.2a). The parent drug itself is pharmacologically inactive (Inturrisi et al. 1983), but all the major metabolites (bar morphine-3-glucuronide) are active to various extents and have different pharmacokinetic profiles. There are therefore multiple factors to consider when selecting a hapten or haptens for a heroin vaccine. A strategy targeting heroin would attempt to sequester the parent before it could be hydrolyzed, but should sequestration by the antibody not be fast enough, this would result in an ineffective vaccine. A hapten targeting 6-AM, the more stable first metabolic intermediate exhibiting the initial psychoactive pharmacology, may therefore be a more realistic and effective strategy. An important consideration is that heroin can rapidly pass through the blood–brain barrier (BBB), more so than its metabolites, potentially escaping the clutches of protective antibodies circulating in the plasma prior to its hydrolysis to the active species (Janda and Treweek 2012). In fact, however, antibody binding is believed to occur on a much more rapid timescale than brain localization (Orson et al. 2014). Morphine could also be considered an important target since it is the first stable, long-lived intermediate, with a much higher plasma exposure than both heroin and 6-AM (Cook et al. 1993). However, due to its higher polarity and reduced brain penetration, the concentrations of morphine observed in the brain are more likely a result of hydrolysis of heroin and/or 6-AM within the brain rather than BBB passage of morphine itself; in this event, sequestering morphine in the bloodstream may not substantially alter the brain concentration of morphine, and no significant modulation of behavioral effects would be achieved (Seleman et al. 2014).
Janda and Koob attempted to delineate the most favorable drug target for an anti-heroin vaccine through direct comparison of heroin and morphine haptens (Schlosburg et al. 2013) (Fig. 18.3). Vaccination with Her resulted in a substantial increase in binding of both heroin and 6-AM in the blood, thus reducing the amount of unbound drug able to enter the brain, but essentially no binding of morphine was seen, whereas Mor vaccination resulted in selective morphine sequestration. Supporting this data, Her has been shown to elicit antibodies against both heroin and 6-AM, but not towards morphine, suggesting that Her presents only heroin- and 6-AM-like epitopes to the immune system (Bremer and Janda 2012). In key behavioral studies, Her elicited protection against heroin-induced antinociception, but no significant effect was observed for Mor-vaccinated rats. These data taken together strongly suggest that although protection against heroin is important, protection against 6-AM appears to be a key driver in obtaining overall protection against the effects of heroin, and morphine-selective vaccines are relatively ineffective.
18.1.2 Oxycodone
The choice of hapten for oxycodone is much more straightforward than heroin. There is only one major metabolite, noroxycodone, which is not believed to contribute to the pharmacological effects in humans (Kim Pei and Smith 1994; Lalovic et al. 2006) (Fig. 18.2b). A key study used pharmacokinetic–pharmacodynamic modeling to show that pupil constriction in humans, a centrally mediated effect, is fully attributable to levels of the parent oxycodone. However, noroxycodone still has significant plasma exposure, and therefore, selectivity of the antibodies for the parent drug may be important to achieve maximal efficacy.
18.1.3 Cocaine
Although cocaine has similarly unstable ester functionalities as heroin, its hydrolysis is slower (Schramm et al. 1993) (Fig. 18.2c). The major metabolites, benzoyl ecgonine and ecgonine methyl ester, are believed to have limited psychoactivity, although there is some debate over whether benzoyl ecgonine is responsible for some of the effects of cocaine administration (Morishima et al. 1999; Brust 2004). However, since benzoyl ecgonine has much greater plasma exposure than cocaine, accumulating over time due to its long half-life (Ambre et al. 1988), this species has the potential to limit binding of nonselective antibodies to the parent drug, especially after repeated cocaine administration. It therefore may be important to develop an anti-cocaine immune response that is selective for cocaine (Schramm et al. 1993).
One other complicating factor is that abuse of cocaine with alcohol has been shown to result in an additional metabolite, cocaethylene, resulting from transesterification of the methyl ester with ethanol (McCance et al. 1995). This metabolite is also psychoactive and results in longer-lasting effects than when cocaine is taken alone. This may therefore be an important practical consideration when predicting the selectivity of antibodies generated by an anti-cocaine vaccine that will be required for efficacy beyond the clinic.
18.1.4 Nicotine
Nicotine is much more stable than heroin or cocaine, with only one major metabolite, cotinine (Hukkanen et al. 2005; Yuki et al. 2013). Although it possesses some psychoactive properties, cotinine is a much weaker agonist of acetylcholine receptors (Dwoskin et al. 1999) and appears to have no pharmacological effect at feasibly attainable levels in the blood after smoking (Hatsukami et al. 1997) (Fig. 18.2d). However, cotinine can antagonize the effects of nicotine (Keenan et al. 1994) and has comparatively higher serum concentrations due to a substantially longer half-life (Yuki et al. 2013). A nonselective vaccine would therefore likely be less efficacious than a vaccine that was selective for nicotine.
Conversely, it has been suggested that this antagonistic activity of cotinine may actually make it a viable target for an anti-smoking vaccine as the presence of cotinine increases withdrawal during nicotine replacement therapy (NRT) (Oliver et al. 2007). However, given that maintained abstinence from nicotine is required for complete smoking cessation, increasing its pharmacological efficacy would appear to be a risky approach, and nicotine itself is the more popular target of anti-smoking vaccination. In any case, it appears crucial to obtain antibody selectivity for one of these two molecules.
18.1.5 Methamphetamine
Methamphetamine, like nicotine, is relatively stable, with amphetamine being the only appreciable metabolite (Schep et al. 2010; Barceloux 2012) (Fig. 18.2e). Although amphetamine is also psychoactive, it only reaches relatively low plasma concentrations and therefore has a negligible effect following methamphetamine administration (Perez-Reyes et al. 1991; Cook et al. 1993). Methamphetamine therefore represents the simplest scenario for hapten choice, as neither targeting multiple species nor obtaining selectivity for the parent drug is likely necessary to achieve high efficacy.
18.2 Structure Modification for Linker Introduction
The structure of the target drug must inevitably be modified by the attachment of the linker that covalently connects it to the requisite carrier protein or peptide. As discussed above, modification of the structure should ideally be achieved without significantly impacting the antibody recognition of the drug, thus avoiding generation of antibodies that may have high affinity for the hapten but poor affinity to the soluble drug. This is theoretically achieved by the strategic position of the linker at a distal location on the drug to the optimal epitope, although in practice, the latter is difficult to predict and define. Given that antibody binding is a result of multiple molecular interactions with polar and hydrophobic surfaces, multiple attachment points may need to be tested in order to identify the optimal linker location. Furthermore, the synthetic complexity must also be considered; if the resulting hapten is fairly intractable to synthesis, it may preclude its therapeutic use even if it demonstrates high efficacy.
The previous section illustrated that the stability and/or metabolic lability of the drug is another key factor. In the case of heroin, there are multiple, rapidly formed metabolites that the immune response may need to be directed against in order to block the effects of the drug. However in the case of nicotine, the primary metabolite cotinine is essentially inactive yet is present at much higher concentrations in the blood after smoking (Hukkanen et al. 2005), and thus, a linker position ideally should be selected to provide an immune response targeted specifically towards the parent drug.
There are several positions on each drug structure that have been commonly used for linker attachment (Fig. 18.4). The majority of these were chosen due to ease of synthetic derivatization, although stability and epitope presentation are also key considerations.
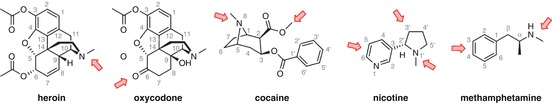

Fig. 18.4
Commonly used sites for linker attachment
18.2.1 Heroin
Heroin and cocaine are rigid molecules, with arguably three key chemical motifs that may comprise the critical pharmacophore for antibody recognition: the bridgehead nitrogen and the two esters. Derivatization of these functional groups is far less synthetically challenging than alternative strategies given that syntheses of haptens for these drugs start from the corresponding naturally derived precursors, and thus, the majority of heroin and cocaine haptens link off of these positions.
As discussed previously, heroin hydrolyzes to give psychoactive metabolites 6-AM and morphine, and obtaining effective protection following heroin administration appears to require antibodies that are directed towards both 6-AM and heroin. Given the comparatively larger size and rigid three-dimensional shape of heroin compared to other drugs of abuse, it can be argued that heroin can display multiple chemical epitopes to the immune system (Matyas et al. 2014). It therefore follows that haptens linked through different locations should provide alternative faces of the molecule as the binding epitope and thus generate antibodies with differing selectivity. However, exploration of alternative linker positions on heroin has been limited, with all examples utilizing stable linkage through the bridgehead nitrogen (Schlosburg et al. 2013; Bremer and Janda 2012; Matyas et al. 2014; Li et al. 2014). A wider variety of linking positions have been explored for morphine haptens; derivatization of the bridgehead nitrogen appears to elicit the most selective anti-morphine antibodies, with antibodies with equivalent affinities towards heroin, 6-AM, and morphine being raised against haptens linked through the 6’ alcohol (Stowe et al. 2011).
18.2.2 Oxycodone
Oxycodone mostly shares the structural considerations discussed for heroin, but its pharmacokinetic profile is much simpler, with only the parent drug showing appreciable activity. Again, only one linkage position has been explored for haptens targeting oxycodone, namely, by derivatization of the C6 ketone (Pravetoni et al. 2012b, 2013). One could envisage that linking through the bridgehead nitrogen, thereby presenting the ketone as part of the epitope, may provide greater selectivity over heroin and morphine, although as will be discussed in Sect. 18.6.2 that is not necessarily desired.
18.2.3 Cocaine
As with heroin, there are three functional motifs that can potentially provide strong interactions with an antibody. Linking through the bridgehead amine has been most extensively investigated for cocaine (Cai et al. 2013a, b, c; Fox et al. 1996; Ramakrishnan et al. 2014), but derivatization of the C2 ester has also been explored (Carrera et al. 1995, 2001; Cai et al. 2013b).
Janda carried out a comparative study of two positions for cocaine hapten linkage (Cai et al. 2013c): attachment to the protein via the C2 ester in GNC (Carrera et al. 1995), a hapten known to elicit antibodies that result in a protective effect, or through the bridgehead nitrogen in GNNS, a linkage position previously shown to be effective in a parallel catalytic antibody approach (see Sect. 5.11) (Matsushita et al. 2001) (Fig. 18.5). Both haptens elicited a comparable immune response, with GNC generating slightly higher-affinity antibodies, albeit at a slightly lower concentration. GNE and GNNA, two alternative haptens which utilize the same linker positions as GNC and GNNS, respectively, but are stabilized through bioisosteric replacement of the C2 ester, gave similar results, albeit with the nitrogen-linked hapten giving slightly higher-affinity antibodies at a lower concentration. These results suggest that there is no real preference for linker location between these two positions in conferring greater immunogenicity. However, GNE was shown to grant greater protection than GNNA (and GNC) upon cocaine-induced psychomotor stimulation, which could suggest that antibody concentration may be the crucial factor is eliciting an effective anti-cocaine immune response and thus linker location may not have a significant impact. Alternatively, there was no data obtained for the selectivity of the antibodies raised for cocaine over benzoyl ecgonine, which may be the key to understanding the superior protective effect displayed for GNE.
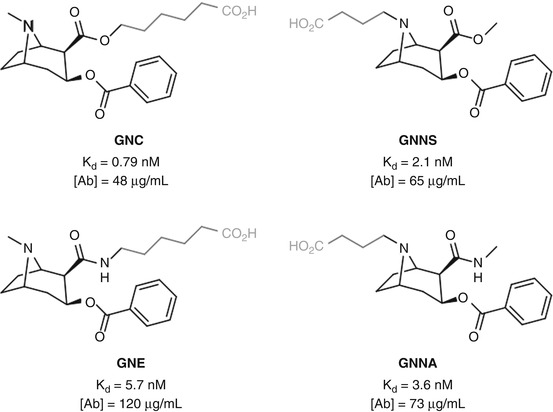

Fig. 18.5
Janda’s investigation of C2-linked and bridgehead nitrogen-linked cocaine haptens (Cai et al. 2013c)
18.2.4 Nicotine
The simple molecular structure of nicotine affords few potential binding interactions for the generation of high-affinity antibodies (relative to cocaine and the opioids), and this is a primary concern when attempting to determine an innocuous linker attachment position. Multiple locations have therefore been investigated, including the pyrrolidine amine (Meijler et al. 2003; Pravetoni et al. 2012a), the 3’-position on the pyrrolidine ring (Langone et al. 1973; Pentel et al. 2000), and various positions on the aromatic ring (Hieda et al. 1997; Pryde et al. 2013; de Villiers et al. 2010, 2002).
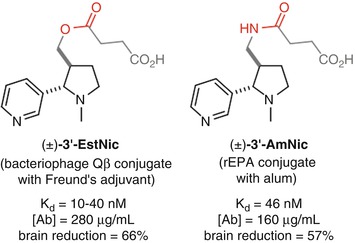
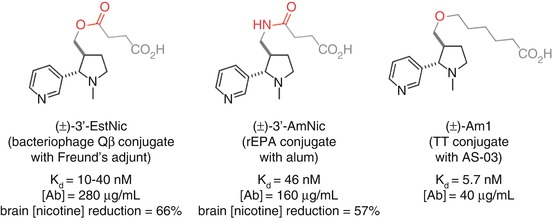
Conjugation through the 3’ position has been investigated with Langone’s (±)-3’-EstNic (Langone et al. 1973) hapten and Pentel’s (±)-3’-AmNic hapten (Pentel et al. 2000) (Fig. 18.6). Both demonstrated the ability to elicit a robust immune response with moderate affinity antibodies at high concentrations that were able to promote a reduction of nicotine concentrations in the brain (Cerny et al. 2002; Maurer et al. 2005; Pentel et al. 2000; Hieda et al. 2000; Pravetoni et al. 2011). These haptens result in antibodies with high selectivity over cotinine, as would be expected due to the distal location of the linker from the site of oxidation. However, it is possible that the proximity of the linker to the ring junction may perturb the structure from the natural conformation, which would render this position suboptimal for the generation of high-affinity antibodies.
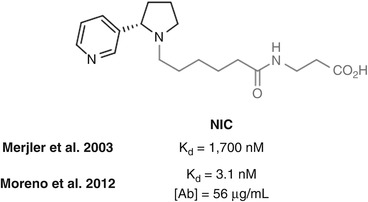
Janda has investigated linking from the pyrrolidine nitrogen using NIC, which in one study in mice was found to elicit poor affinity anti-nicotine antibodies (Meijler et al. 2003) (Fig. 18.7). However, in a repeat study in rats using a different adjuvant and different schedule, (±)-NIC elicited very high antibody affinities for nicotine (Moreno et al. 2012). Unfortunately, only limited behavioral effects were observed, possibly a result of the low antibody concentration elicited. It would be anticipated that the close proximity of the linker to the position of the additional carbonyl group that differentiates cotinine resulted in antibodies that were unselective for nicotine over its metabolite. However, in a different study, mAbs raised against NIC showed no cross-reactivity with a cotinine hapten also linked at the pyrrolidine nitrogen (Isomura et al. 2001), suggesting that selectivity is possible using this linkage position.
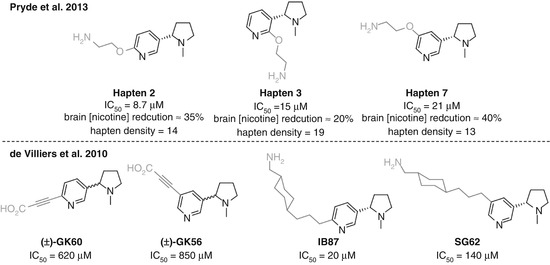
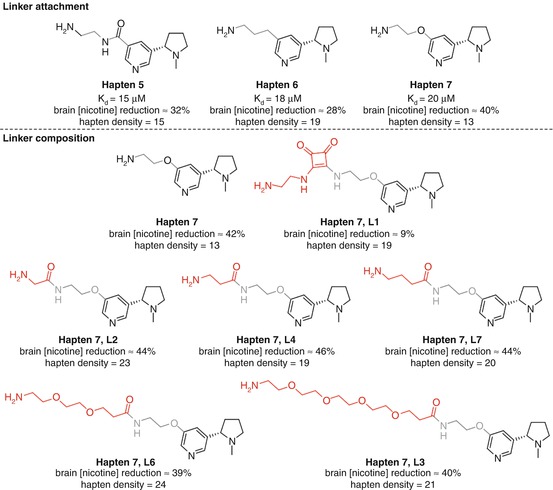
McKluskie investigated a range of nicotine haptens linked at different positions on the pyridine ring (Pryde et al. 2013) (Fig. 18.8a). Haptens 2, 3, and 7 from that study allowed direct comparison as they obtained similar hapten densities and all utilized the same linker strategy. Competitive ELISA data suggested that the C6-linked Hapten 2 was slightly superior, with C5-linked Hapten 7 showing the lowest affinity to nicotine of the three. However, Hapten 7 was able to reduce brain concentrations of nicotine to a greater extent than Hapten 2, which was in turn superior to Hapten 3. This is the first example discussed that demonstrates a common hazard in small molecule vaccine research. The antibody affinities measured by competitive ELISA are dependent on the serum binding to the hapten, not the soluble drug, and thus do not necessarily give a true indication of the vaccine efficacy. The direct measurement of nicotine brain concentrations is a simple yet powerful method to assess how much is actually being sequestered in the bloodstream, and this study therefore suggests that linking off the C5 position can give more effective anti-nicotine protection than either the C2 or C6 positions, due to either spatial or electronic effects. The poor results for Hapten 3 could potentially be due to the close proximity of the linker to both the pyrrolidine and pyridine nitrogens, shielding the only two functionalities in the nicotine molecule able to form strong and specific interactions with antibodies.
In the work of de Villiers, a similar trend was observed for affinities to nicotine, again using competitive ELISA, but the situation was less clear when analyzing vaccine impact (Fig. 18.8b) (de Villiers et al. 2010). C6-linked GK60 showed a reduction in nicotine-induced dopamine overflow, whereas vaccination using GK56 actually showed an increased response. However, the reverse was seen when comparing SG62 and IB87, where the C5-linked hapten appeared superior.
The disparity in results between the groups of nicotine haptens linked through the pyridine ring may be a result of the effect of the linker on immunogenicity. However, the difficulty in rationalizing this disparity demonstrates a consistent issue with hapten design; experimental testing against metabolites is ultimately required to understand relative efficacy.
18.2.5 Methamphetamine
Methamphetamine is the simplest of all the major drugs of abuse, with a flexible structure and only one major chemical motif: the secondary amine. This could therefore be considered the toughest drug against which to develop high-affinity antibodies. Many positions on methamphetamine have been derivatized for conjugation to carrier proteins, albeit mostly for the generation of mAbs in passive immunization approaches.
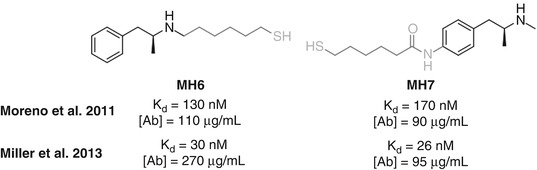
Attachment sites that have been examined for active vaccines include the N-methyl group, simply performed through direct derivatization of amphetamine (Moreno et al. 2011; Miller et al. 2013) and the amine itself, to form a 3° amine (Collins et al. 2014) or amide (Shen et al. 2013), and functionalization of the aromatic ring at both the meta– (Carroll et al. 2011) and para-positions (Byrnes-Blake et al. 2001; Moreno et al. 2011; Duryee et al. 2009) (Fig. 18.4). All the haptens tested have shown some efficacy; however, there are currently few comparative studies between haptens directed towards methamphetamine.
Janda directly compared MH6 and MH7, haptens conjugated through the N-methyl group and the para-position of the aromatic ring, respectively (Fig. 18.9). An initial study in mice showed that both haptens elicited similar concentrations of antibodies with similar affinities. In rats using a different vaccination schedule, comparable affinities were again observed, but MH6 was found to elicit higher antibody concentrations, resulting in a reduction in locomotor activity that was not observed for MH7.
18.3 Linker Composition
Once the optimal position for the linker attachment to the hapten has been identified that minimizes any negative effect on antigen presentation, the composition of the linker must also be considered as an important factor that can affect the immune response. Firstly, a stable linkage is a necessity to avoid loss of the hapten from the carrier protein during both preparation and vaccination. Secondly, in order to achieve effective presentation in the MHCII complex and recognition by B cell receptors (BCRs), it is vital that the hapten be spatially displayed in an effective manner, and the nature, length, and rigidity of the linker are likely crucial components in achieving this. The choice of linker will likely also impact the preparation of the carrier–hapten vaccine by determining the conjugation efficiency and potentially even the tertiary structure of the modified protein. Finally, it is of course vital that the linker not be part of the epitope and contribute to the binding of the resultant antibodies; this additional unwanted immunogenicity will likely produce greater affinity to the hapten at the expense of the target soluble drug.
18.3.1 Nicotine
In the case of Pentel’s nicotine vaccine CMUNic, the antibodies elicited bound the hapten with substantially higher affinity than nicotine, likely due in part to the choice of a urea-containing linker (Hieda et al. 1997) (Fig. 18.10). Ureas are conformationally rigid and display a powerful hydrogen-bonding pattern for potential recognition as an epitope. Even so, the vaccine was still able to demonstrate efficacy, altering the blood–brain distribution of nicotine by partial sequestration in the blood (Hieda et al. 1999).
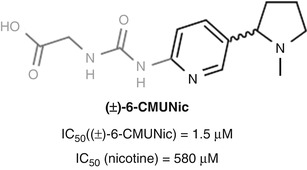

Fig. 18.10
Pentel’s (±)-6-CMUNic hapten elicited preferential binding of the hapten over nicotine (Hieda et al. 1997)
Originally developed by Langone for the development of antibody-based diagnostic assays for nicotine (Langone et al. 1973), (±)-3’-EstNic has become one of the most widely used haptens for nicotine vaccines (Fig. 18.11). It had been found to effectively reduce nicotine concentrations in the brain (Cerny et al. 2002; Maurer et al. 2005) and was progressed into human clinical trials. However, ultimately (±)-3’-EstNic was found to elicit a highly variable response, only delivering long-term abstinence in the limited number of individuals that obtained high antibody titers (Maurer et al. 2005; Cornuz et al. 2008). (±)-3’-EstNic contains a potentially labile ester linkage, and one hypothesis was that this could result in uncontrolled and unpredictable hydrolysis, reducing hapten density and vaccine efficacy. Pentel sought to stabilize the hapten using a bioisosteric amide, giving (±)-3’-AmNic, which was again able to effectively reduce nicotine concentrations in the brain (Pentel et al. 2000; Hieda et al. 2000; Pravetoni et al. 2011). However, the undesirably high variability between individuals was still observed in clinical trials (Hatsukami et al. 2005, 2011; Wagena et al. 2008).
The proximity of functionality within the linker to the intended drug epitope may also be a source of additional, unwanted immunogenicity. Janda therefore developed (±)-AM1, a version of the hapten with an ether linkage that is both stable and should exhibit minimal unwanted immunogenicity. (±)-AM1 was shown to elicit antibodies with high affinity for nicotine, but at a much lower concentration, and an increase in self-administration relative to unvaccinated mice suggests that the protective effects were surmountable (Moreno et al. 2010).
Although it can be rationalized that AM1 should result in antibodies with reduced linker-derived immunogenicity and thus elicit antibodies with greater affinity to the target nicotine, the three 3’-linked nicotine haptens have not been directly compared. Given the huge disparity between the methods employed in the studies performed, there is no experimental data to determine which is superior. As alluded to previously, this is an ongoing issue within the drugs of abuse field, and unless direct comparison is performed, it is not possible to actually test the hypotheses that inspire the design of the next generation of haptens.
As before, it is possible to extract interesting comparative data from the rigorous study conducted by McKluskie, this time analyzing the properties of linkers attached to C5-modified nicotine haptens (Pryde et al. 2013) (Fig. 18.12a). All three haptens with similar hapten densities elicited antibodies that showed similar affinities for nicotine using competitive ELISA, but Hapten 7, appended via an electron-donating ether linker, showed greater efficacy in reducing brain concentrations of nicotine than both electron-withdrawing amide Hapten 5 and alkyl-linked Hapten 6. The effect of the linker composition was then further investigated through modification of Hapten 7 (Fig. 18.12b). This revealed that the amide-containing linkers L2, L4, and L7 gave comparable reduction in brain concentrations of nicotine to the original linker in Hapten 7 and PEG linkers in L6 and L3 only conferred a marginal decrease in efficacy. The conformationally rigid squaramide linker in L1 proved to substantially decrease the ability to reduce nicotine concentrations in the brain, suggesting that flexibility may be required for effective epitope recognition.
18.3.2 Cocaine
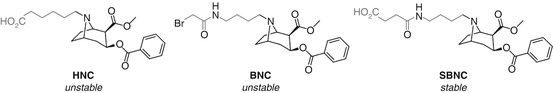
Orson’s use of bridgehead amine-linked cocaine haptens HNC, BNC, and SBNC serves to demonstrate that the linker composition can have a distinct and unexpected impact on the chemical stability (Ramakrishnan et al. 2014) (Fig. 18.13). HNC and BNC showed substantial C2-ester hydrolysis during synthesis and thus elicited an immune response skewed towards benzoyl ecgonine, whereas SBNC apparently remained intact during synthesis and was able to elicit antibodies that showed no appreciable binding to benzoyl ecgonine (discussed in more detail in Sect. 18.7.2, Fig. 18.26). It is difficult to rationalize this improved stability given the minor structural differences between BNC and SBNC, and as such, this does not appear to be a general solution even within the cocaine vaccine field, but this study does demonstrate that the linker composition may have a greater impact on the hapten than anticipated.
18.3.3 Peptidic Linkers
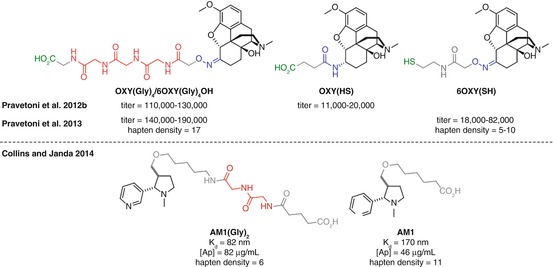
The biological processing that results in MHCII presentation of an immunogen requires a peptidic substrate. As discussed previously, since drugs of abuse antigens are not peptidic, they require conjugation to a carrier protein or peptide. It would therefore follow that a more peptidic conjugate vaccine might facilitate efficient binding within the MHCII complex and recognition by the immune system. Despite this, linkers used for protein conjugation are predominantly composed of hydrophobic aliphatic chains, presumably to allow minimal immunogenicity, good flexibility, and ease of synthesis, which by the logic above may negatively impact on binding to the MHCII protein. Increasing the peptidic nature of the linker may therefore be expected to increase the immune response to the attached hapten, and this hypothesis has begun to be explored. The use of polyglycine linkers, first investigated in vaccinations against Bacillus anthracis (Schneerson et al. 2003; Kubler-Kielb et al. 2006), has been employed in both opioid and nicotine vaccines (Pravetoni et al. 2012b, 2013; Collins and Janda 2014).
Pentel found that the introduction of a tetraglycine unit in an oxycodone vaccine, OXY(Gly) 4 , elicited substantially higher anti-oxycodone antibody titers than the corresponding hapten OXY(HS) (Pravetoni et al. 2012b) (Fig. 18.14a). However, the use of different linker attachment chemistry in the two haptens may have been partially responsible for this effect. Later work by Pentel used both hapten linkers with the same linking chemistry in both oxycodone and hydrocodone vaccines, demonstrating for the tetraglycine analogs both higher titers and reduced brain concentrations of oxycodone in vaccinated animals (Pravetoni et al. 2013) (Fig. 18.14b). However again a direct comparison cannot be made since, in this case, the tetraglycine haptens were conjugated through amide bond formation, whereas the comparators were linked to the carrier protein through maleimide conjugation, resulting in substantially lower hapten densities. The tetraglycine linker was reported by Pentel to be more effective than the corresponding diglycine or non-peptidic linker for a morphine vaccine, although no comparative data to support this conclusion was shown (Pravetoni et al. 2013).
In a more closely controlled example, the application of a diglycine linker to a nicotine vaccine was carried out by Janda (Fig. 18.14c) (Collins and Janda 2014). An increase in both antibody concentration and affinity was observed for the diglycine-containing hapten AM1(Gly) 2 than corresponding hapten AM1. Although the structures and conjugation chemistry were the same in this case, there was still a disparity in hapten densities; however on this occasion, there were fewer copies of hapten in the superior vaccine. Given that it is generally considered that higher hapten densities correlate with increased immune response (Marco et al. 1995), this data strongly suggests that the AM1(Gly) 2 hapten elicits a superior response due to the inclusion of the diglycine motif.
Further work will determine the optimal length and composition of peptidic linkers based on these promising initial findings using di- and tetraglycine, providing a modular and generally applicable solution to increase the efficacy of vaccines against any drug of abuse. However, the inconsistent hapten densities obtained within these studies attempting to directly compare linkers again highlight the difficulties in performing a controlled comparison of hapten or linker structure.
18.4 Choice of Conjugation Method
Another important design element for the linker is the functionality through which the hapten will be attached to the carrier. It is crucial that the linkage be stable and use orthogonal conjugation chemistry to avoid side reactions with either the carrier or the hapten itself. The two most frequently utilized conjugation methods append the hapten to surface-exposed lysine residues, which are abundant in carrier proteins and peptides. Direct amide bond formation requires the activation of a carboxylic acid hapten, usually using EDC/NHS chemistry (Fig. 18.15a). This requires that there are no other carboxylic acids in the hapten or reactive amines with which the resulting activated ester can couple. The stability of the activated intermediate (“OAct” in Fig. 18.15a) can potentially be tuned to give the desired reactivity.
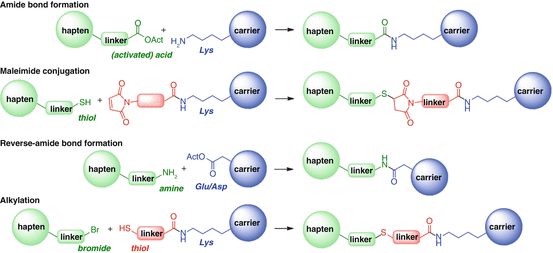

Fig. 18.15
Standard methods of hapten–protein conjugation. “OAct” refers to the activated ester intermediate, usually an N-hydroxysuccinimide ester or equivalent
Alternatively, the protein lysine residues can be functionalized for reaction with a thiol-containing hapten by initial coupling with a maleimide-containing activated carboxylic acid (Fig. 18.15b). The thiol–maleimide conjugate addition is rapid and highly orthogonal to other functionality. However, it has been observed that maleimide conjugation tends to give lower hapten densities than the corresponding amide bond formation (Collins et al. 2014). The requirement for a linker between the carrier and the maleimide introduces additional chemical matter between the hapten and the carrier, potentially affecting the resulting immune response. Furthermore, since conjugation of thiols to maleimides is usually not quantitative, a surfeit of unreacted linker–maleimide appendages will be decorating the protein surface, possibly proving detrimental to stability and immunological efficacy. The precursor thiols are also unstable, forming disulfides upon storage. Although this can be reversed by performing the conjugation reaction under reductive conditions, this is potentially incompatible with carrier proteins containing disulfide bridges. Finally, it has been shown that thiol addition to maleimide can be reversible, leading to unexpected loss of the hapten from the carrier (Fontaine et al. 2014).
Less frequently, the reverse amide bond formation is performed for hapten conjugation (Fig. 18.15c). It is possible to conjugate to the intrinsic surface aspartate and glutamate residues, but the lysines can also be modified to incorporate additional carboxylic acids for conjugation to an amine-containing hapten, usually using succinic anhydride. However, analysis post-conjugation can be complicated by a side reaction resulting from the activation of the acids, namely, irreversible O-acylisourea to N-acylurea rearrangement (DeTar and Silverstein 1966), which results in an increase in protein mass without concomitant hapten conjugation.
Rarely, bromide-derivatized haptens can be used in combination with carrier proteins that have been decorated with thiols through addition to the protein lysine residues (Duncan et al. 1983) (Fig. 18.15d). Although this alkylation should be orthogonal to other functionalities that may be in the hapten, there is potential to introduce disulfide bridges both intra- and intermolecularly, which can complicate the resulting conjugates.
Other types of conjugation method have been explored, including the production of squaric acid diamides from the corresponding hapten amines and lysines (Tietze et al. 1991), Huisgen [3 + 2] cycloadditions from azides and terminal alkynes (Rostovtsev et al. 2002; Tornøe et al. 2002; Agard et al. 2004), traceless Staudinger reactions from azides and esters (Grandjean et al. 2005), and oxime ligations from hydroxylamines and aldehydes (Ulrich et al. 2014); these may be required where standard conjugation chemistry is incompatible with the functionalities in the haptens. These alternative approaches are rarely utilized in drugs of abuse vaccines; the chemistry involved is often more complicated, the linkages can be less stable, and some require the use of relatively bulky or functionalized linkers which would be considered too intrusive for conjugation of small molecules with low inherent size and immunogenicity.
18.5 Multivalent Haptens
Combination vaccines are well established in the arena of infectious diseases, either using multiple immunogens for the same target (e.g., pneumococcal disease vaccines) or multiple unrelated immunogens, for example, against measles, mumps, and rubella (MMR) or diphtheria, pertussis, and tetanus (DPT). In these cases, no compromising effects on the response to the individual immunogens have been observed (Skibinski et al. 2011; Andrews et al. 2012). Combination vaccines for drugs of abuse have therefore been explored as a potential solution to problems of poor selectivity and efficacy.
18.5.1 Multivalent Haptens Targeting Multiple Species
As discussed, designing an efficacious hapten to target the complex family of heroin metabolites has significant challenges, and targeting multiple species may prove to be the most effective strategy. Co-vaccination using haptens that mimic multiple species is an alternative strategy that may give superior results.
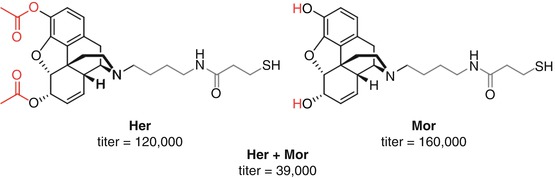
Janda investigated co-vaccination of the two haptens, Her and Mor, that had shown effective stimulation of heroin and anti-6-AM and anti-morphine antibodies, respectively (Stowe et al. 2011) (Fig. 18.3). Given the rapid hydrolysis of heroin to morphine and its greater AUC than heroin and 6-AM, it was proposed that the addition of anti-morphine antibodies to those generated by Her may elicit enhanced protection following heroin administration (Cook et al. 1993) (Fig. 18.16). However, the co-vaccination of both Her and Mor haptens appeared to drastically affect the overall immune response, resulting in a far lower antibody titer and reduced protection against the antinociceptive effects of heroin when compared to Her alone, with no prevention of heroin self-acquisition. However, this combination Her/Mor vaccine still resulted in more significant heroin-induced nociceptive protection than Mor alone despite the substantially reduced antibody titers, strongly suggesting that raising a sufficient concentration of anti-heroin and anti-6-AM antibodies is required for protection against heroin abuse. Furthermore, the reduction in total antibody response suggests that co-vaccination with these two opioid haptens compromises the immune response towards both haptens (Stowe et al. 2011).
18.5.2 Multivalent Haptens Targeting Multiple Drugs
A successful targeted drug of abuse vaccine, able to fully and specifically sequester the desired drug, may still prove inadequate to prevent drug user relapse due to ready availability of related drugs. It is therefore worthy to pursue the development of vaccines that sequester multiple related drugs of abuse.
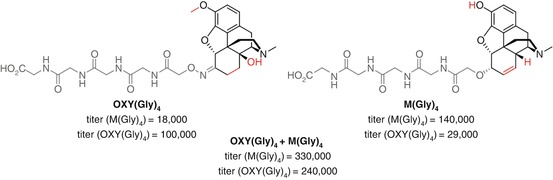
Heroin and oxycodone are current opioid vaccine targets which to date exhibit limited cross-reactivity in their antibody responses. Pravetoni investigated the potential of co-vaccination using oxycodone and morphine haptens OXY(Gly) 4 (Pravetoni et al. 2012b, 2013, 2014a, 2014b) and M(Gly) 4 (Raleigh et al. 2013) that had previously been shown to elicit protective effects against their respective opioids in multiple animal models (Pravetoni et al. 2012c) (Fig. 18.17). The divalent vaccine elicited significantly higher titers towards each opioid than the individual vaccines. Furthermore, a greater increase in serum retention was observed for both 6-AM and oxycodone by the divalent vaccine than either monovalent vaccine independently, although vaccination with M(Gly) 4 resulted in the lowest brain concentrations of morphine. One caveat in interpreting these results is that the total amount of conjugate administered for the divalent vaccine is double than for the monovalent vaccines, which is likely partially responsible for the raised titers and increased behavioral effects. Furthermore, unconjugated KLH was added to the monovalent vaccine to keep the total protein injected constant. In the case of a bivalent nicotine vaccine, this has been shown to result in significant reduction in both ELISA titer and drug serum retention, likely due to immune system diversion towards the highly immunogenic protein (de Villiers et al. 2013). Furthermore, although ELISA data demonstrates that a limited proportion of the antibodies bind to both haptens, this may not be the case for the soluble opioids. Overall, however, this study does suggest that co-vaccination with multiple haptens targeting different opioids can be an effective strategy.