Chapter 8 Handling, safety and practical applications for use of essential oils
INTRODUCTION AND BACKGROUND
A knowledge of the chemical and physical properties of the essential oils and materials used gives a logical background that can inform and guide in their applications in aromatherapy. The everyday storage, handling and use of oils with clients involve an underlying understanding of safety coupled with an awareness of the ever-increasing burden of legislation. The basics of anatomy and physiology appropriate to aromatherapy and the links to psychological well-being also need to be considered within this context. Topics in this chapter draw directly on the concepts previously covered in analysis and composition of oils. Important sources of information about properties and handling of essential oils can be found in established literature, in specialist journals and from the individual Safety Data Sheet for that oil. Safety Data Sheets cover areas including composition, health hazards, first aid procedures, dire and explosion hazard, procedures for accidental release, handling, storage, ecological implications, transport, labelling, regulations and other information. The oil supplier should have Safety Data Sheets for the materials they sell. An example for Eucalyptus globulus is shown in this chapter, and in the previous chapter we saw such sheets for rosemary and lemongrass, where they were linked with analytical data.
STORAGE OF OILS
Closures
The container must be sealed by a closure. If air is allowed to enter and interact with the essential oil, the chemical reaction of oxidation can occur. Oxidation in this case can be considered the addition of oxygen to an oil constituent to form a new compound. New compounds formed will alter the composition of the oil. Water vapour may also enter from the air. An open or incompletely sealed container will allow essential oil components to escape as vapours, and this will change the balance of constituents. The best choice of closure for a bottle is a screw cap fitted with a wad or washer. Ideally bottles are fitted with childproof tops and drop dispensers to control amounts dispensed.
Temperature
It is important to store essential oils under cool conditions. Woody oils such as Cedrus atlantica (cedarwood), Santalum album (sandalwood), Pogostemon cablin (patchouli) and Vetiveria zizanioides (vetivert) can be stored at a low room temperature, no higher than 15 °C. Resinoids can be kept at low temperatures, no higher than 10 °C, as may be found in a cellar. All other oils are best stored at temperatures found in a typical domestic refrigerator at around 5 °C. Rose otto and rose absolute and a few other oils may congeal or solidify at low temperatures, but re-melt at room temperature. It is important that they are allowed to do this gradually without application of artificial heat.
LABELLING
From practical considerations, the actual size of the label on a typical essential oil bottle is very small. This will limit the amount of information it can carry. There are a number of recommendations and guidelines for labelling from various regulatory and professional bodies, which need to be applied with certain legislative requirements in mind. These include guidance from AOC (Aromatherapy Organizations Council), IFRA (International Fragrance Association) and ISO (International Organization for Standardization). The EC regulations are explained in Chapter 7.
AROMAFACT
Oils that are bought directly from a supplier or through a retail outlet will probably carry most of the information listed above. However, you may supply an essential oil, or a blend of oils that is specifically formulated for use with an individual client. This will need additional labelling and must link to your therapists’ record-keeping system. These oils will need to have additional information, including the client’s name, date administered, directions for use and any special precautions or interactions. This is similar to the situation for drugs dispensed through a pharmacy. In terms of safety, the abbreviation GRAS on a label means Generally Recognized as Safe.
LEGISLATION AND REGULATORY BODIES
MSDS – Material Safety Data Sheets (now usually referred to as SDS, Safety Data Sheets)
Suppliers, manufacturers and importers who make up the chain of supply of essential oils to the aromatherapist, and ultimately the client, are responsible for drawing up the MSDS. Each time an oil is repackaged or relabelled, a MSDS should be prepared and relevant additional information provided before it is passed on to the next customer in the chain of supply. When a chemical is supplied to the general public in retail outlets, by mail order or as free samples and prizes, a MSDS is only needed if the purchaser intends to use that chemical at work, if the chemical preparation is classified as dangerous for supply (according to the CHIP 2 regulation) or if the purchaser asks for a safety data sheet. For most practical situations the packaging and labelling will supply sufficient information for safe use. The labelling of aromatherapy oils and blends is described in Chapter 7.
A typical MSDS is shown for Eucalyptus globulus in Figure 8.1 and gives a fairly comprehensive range of information about the oil. The example is one provided by a British oil supplier, and shows that a reputable supplier can provide high-quality relevant data. There are a number of features included that are explained elsewhere in this book, i.e. specific gravity, LD50, GRAS, CHIP regulations, flash point, RIFM, hazard symbols, R phrase and S phrase. In addition there are a number of other acronyms that need to be identified.

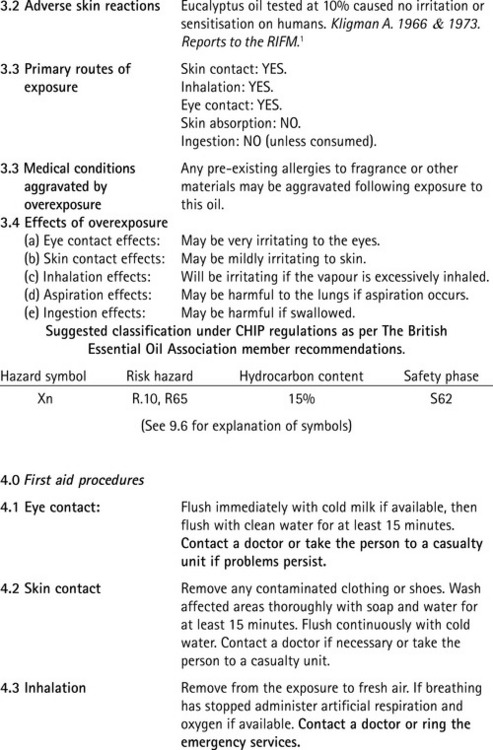




Figure 8.1 A typical material safety data sheet. Courtesy of Medical Aromatherapy Training Services. Supplied by Charles Wells, Essentially Oils and Analytical Intelligence Ltd.
Restrictions on the use of a given ingredient are identified. Restrictions are set out in the Directive itself or in the IFRA (International Fragrance Association) code of practice. These restrictions may take the form of a quantitative limitation (expressed as a percentage of the final product or as a concentration for application to the skin), or the ingredient may have to meet certain specifications or may only be used in conjunction with certain specified ingredients. These substances are marked with one asterisk * for IFRA restrictions or with two asterisks ** for restrictions in the Cosmetic Products Directive.



