Fig. 11.1 Platelets and platelet aggregation.
Subendothelial macromolecules such as von Willebrand factor and collagen interact with glycoprotein receptors (GPVI and GPIb) on platelets, causing activation of platelets and upregulation of GPIIb/IIIa receptors, which are crosslinked by fibrinogen, resulting in aggregation. During the initial processes of aggregation, stimulation of the synthesis and release of a number of platelet-derived substances, such as thromboxane A2 (TXA2) synthesised from arachidonic acid (AA) by cyclo-oxygenase-1 (COX-1), ADP, and other factors (see text) further promote aggregation by upregulation of GPIIb/IIIa receptors. Conversely, prostacyclin (PGI2) from endothelial cells inhibits activation and upregulation of GPIIb/IIIa receptors. Thrombin is generated by the action of factor Xa on prothrombin (see Fig. 11.3).
Extension of the platelet plug requires activation of platelets and their subsequent aggregation together (homotypic aggregation). Platelets are initially activated by exposure to soluble agonists, such as thrombin generated by local coagulation, ADP released from endothelial cells and collagen. These activators lead to an increase in intracellular Ca2+ and activation of (MLCK). MLCK phosphorylates myosin light chains in the platelet which interact with actin, disrupt the platelet cytoskeleton and change the shape of the platelet.
The increase in platelet intracellular Ca2+ activates phospholipase A2, which liberates arachidonic acid (AA) from membrane phospholipids. AA is then converted by cyclo-oxygenase type 1 (COX-1) in the platelet to thromboxane A2, the most potent naturally occurring pro-aggregating agent, which diffuses from the platelet. Significant disruption of the platelet cytoskeleton as the platelet changes shape also initiates a platelet release reaction, which expels mediators in platelet dense storage granules from the cell. Fusion of dense granules with the platelet cell membrane releases platelet factor 4, adrenaline, ADP and serotonin. Outside the platelet ADP, thrombin and thromboxane A2 interact with specific platelet surface receptors and trigger intracellular pathways that express and activate GPIIb/IIIa collagen receptors on the surface of the platelets (Figs 11.1 and 11.2). Therefore, ADP released from platelets acts as a mediator for initiators of platelet activation, such as collagen and thrombin. Secondary irreversible homotypic platelet–platelet aggregation follows platelet activation when the activated GPIIb/IIIa receptors on the surface of the platelets are crosslinked by fibrinogen in the plasma.
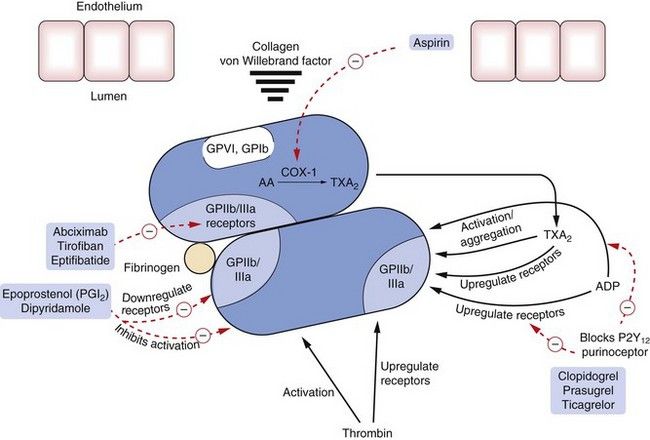
Fig. 11.2 Sites of action of major drugs used in haemostasis.
Drugs act directly or indirectly to inhibit activation of platelets or to block or reduce upregulation of the glycoprotein GPIIb/IIIa receptors (integrin receptor family), which are necessary for aggregation of platelets. Abciximab is an antibody, tirofiban a non-peptide inhibitor and eptifibatide a peptide inhibitor of these glycoprotein receptors. Epoprostenol and dipyridamole inhibit activation of platelets and downregulate the glycoprotein receptors by increasing cAMP. Clopidogrel inhibits ADP receptors and prevents ADP-induced upregulation of the glycoprotein GPIIb/IIIa receptors and platelet aggregation. Aspirin inhibits the generation of thromboxane A2 (TXA2) by cyclo-oxygenase-1 (COX-1), which otherwise causes activation of platelets and upregulation of GPIIb/IIIa receptors. AA, arachidonic acid. For direct and indirect inhibitors of thrombin, see Fig. 11.3.
The substances released from platelet dense granules also facilitate haemostasis by:
 reducing prostacyclin (prostaglandin I2, PGI2) synthesis by vascular endothelium; prostacyclin is a vasodilator and a potent inhibitor of platelet aggregation,
reducing prostacyclin (prostaglandin I2, PGI2) synthesis by vascular endothelium; prostacyclin is a vasodilator and a potent inhibitor of platelet aggregation,Expression of platelet GPIIb/IIIa surface receptors can be inhibited by an increase in the concentration of cyclic nucleotides (cAMP, cGMP) in the platelet. This is the mechanism by which prostacyclin (PGI2) inhibits platelet aggregation (Figs 11.1 and 11.2).
Polyunsaturated (omega-3) fatty acids in fish oils are precursors for thromboxane A3, which causes less platelet aggregation than thromboxane A2; they also increase production of a modified form of prostacyclin (PGI3) by vascular endothelium which has equal anti-aggregatory activity to PGI2. Therefore, a high intake of fish oils creates a state in which platelets are less able to aggregate.
Heterotypic platelet aggregation can also arise when platelets aggregate with leucocytes (and particularly monocytes) in circulating blood. This process has been detected close to atherosclerotic lesions but also in a variety of inflammatory conditions, and may follow initial activation of platelets by vascular damage. Heterotypic aggregation results from expression of P-selectin on the surface of the platelet. P-selectin is one of several molecules found in platelet alpha-granules, which are released when the platelet cytoskeleton is only minimally disrupted by thrombin- or ADP-mediated activation of the cell. Heterotypic platelet aggregation is not inhibited by some antiplatelet drugs (such as aspirin) to the same extent as homotypic aggregation
Blood coagulation and the coagulation cascade
Both coagulant and anticoagulant factors regulate haemostasis. Activation of the coagulation cascade is divided into extrinsic and intrinsic pathways (Fig. 11.3). The factors involved in these cascades amplify the coagulation response and work together to produce a thrombus. The extrinsic pathway accounts for most of the coagulation in vivo, but both coagulation pathways respond to breaches in endothelial integrity much more slowly than platelet aggregation. The following description of the pathways is simplified to identify the key steps at which drugs can modulate coagulation.
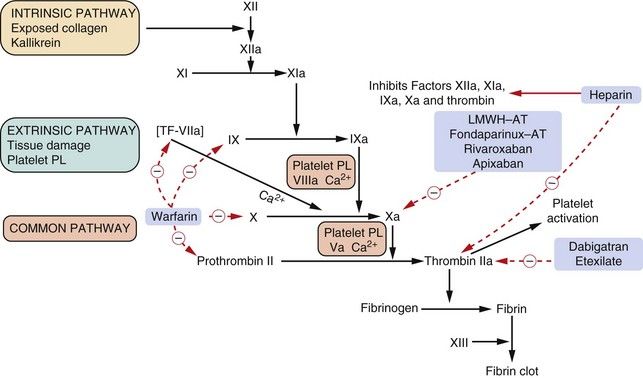
Fig. 11.3 The coagulation cascade and action of anticoagulants.
The complex cascade of clotting factor synthesis is initiated extrinsically by tissue damage. Activation of the clotting factors after damage depends upon platelet factors, tissue factor, phospholipids, Ca2+ and vitamin K. The provision of platelet products is further enhanced by the formation of thrombin, which then activates further platelets as well as causing fibrin formation. Heparin acts at various sites in the cascade by complexing with the anticlotting factor antithrombin III (AT) and inhibiting thrombin (IIa) and the other activated clotting factors shown. Low-molecular-weight heparin (LMWH) complexes with AT but in a different manner to unfractionated heparin and inhibits only factor Xa. Bivalirudin and dabigatran etexilate inhibit thrombin (IIa) action. Warfarin inhibits the synthesis of the vitamin K-dependent clotting factors VII, IX, X and II (prothrombin). Roman numerals indicate the individual clotting factors; PL, phospholipid; TF, tissue factor.
The extrinsic coagulation pathway is initiated by exposure of blood to tissue factor (TF) on the surface of subendothelial cells after vascular injury, and is activated rapidly within minutes of endothelial disruption. Formation of complexes of TF with factor VIIa, and the presence of phospholipids and Ca2+, result in the conversion of inactive factor X to active Xa.
The intrinsic pathway is triggered by contact of blood with a negatively charged surface such as subendothelial collagen, and its activation is delayed by more than 10 min after tissue disruption. The intrinsic coagulation pathway comprises a series of enzyme-mediated reactions involving activation of several clotting factors and eventually activation of factor X.
Activation of factor X, which mediates the hydrolysis of prothrombin to thrombin (factor IIa), is the point at which the two pathways of coagulation converge (Fig. 11.3). The actions of thrombin (factor IIa) and several other activated coagulation factors (Fig 11.3) are inhibited by circulating antithrombin. Antithrombin inhibits coagulation factors after forming complexes with heparin-like molecules that are produced by intact endothelial cells, and with heparin released from mast cells. Once sufficient thrombin has been produced to overcome the effect of circulating antithrombin, the soluble protein fibrinogen is converted to an insoluble fibrin gel. Thrombin also activates factor XIII, which crosslinks the fibrin polymers and forms a fibrin mesh that traps circulating platelets, leucocytes and red blood cells.
Each activated clotting factor is inactivated extremely rapidly so that the coagulation process remains localised at the site of the initiating event. However, in some circumstances, aggregates of platelets combined with fibrin thrombi can embolise and occlude more distal parts of the circulation.
Arterial and venous thrombosis
There are differences in the composition of an arterial or venous thrombus. Arterial thrombosis occurs in the setting of high flow and high shear stress, and platelets play a prominent role in the initiation and growth of the thrombus. In contrast, venous thrombi form in a low-flow, low-shear stress environment. Venous thrombus usually forms initially in the valve pockets of deep veins, and consists mainly of fibrin and red cells with few platelets.
Antiplatelet drugs
Mechanism of action on platelets: The highly potent platelet-aggregating agent thromboxane A2 is formed in platelets from AA by the enzyme COX-1. After release from the platelet, thromboxane A2 acts via TP receptors on the surface of the platelet to generate the intracellular second messengers inositol triphosphate (IP3) and diacylglycerol (DAG). These lead to Ca2+ release in the cell and expression and activation of GPIIb/IIIa receptors.
Inhibition of COX-1 by aspirin reduces platelet thromboxane A2 synthesis and inhibits platelet aggregation, but does not eliminate it completely because other pathways for platelet activation still function (Figs 11.1 and 11.2). Aspirin (acetylsalicylic acid) irreversibly inhibits COX-1 by acetylation (Ch. 29) and since platelets lack a nucleus and cannot synthesise new enzyme, their ability to aggregate will be reduced throughout the lifespan of the platelet. The antiplatelet action of aspirin occurs at very low doses that have little analgesic or anti-inflammatory actions. At higher doses, aspirin also inhibits the production of prostacyclin by vascular endothelium which may offset some of the beneficial effects on platelets. Details of the pharmacology of aspirin can be found in Chapter 29.
Phosphodiesterase inhibitors
Mechanism of action: Dipyridamole has multiple mechanisms of action; the most important is probably inhibition of the reuptake of adenosine by cells. The increased plasma concentration of adenosine promotes vasodilation and inhibits platelet aggregation by stimulation of intracellular adenylyl cyclase and production of the intracellular cyclic nucleotides cGMP and cAMP. Dipyridamole also inhibits phosphodiesterase types 3 and 5, which degrade cyclic nucleotides. High intracellular cyclic nucleotide concentrations inhibit activation of cell surface GPIIb/IIIa receptors, leading to reduced platelet activation (Fig. 11.2). Dipyridamole has a number of other actions that are of uncertain significance, including antioxidant properties.
Pharmacokinetics: Dipyridamole is incompletely absorbed from the gut and is metabolised in the liver. It has a half-life of 12 h. A modified-release formulation is better tolerated than the standard formulation.
ADP receptor antagonists
Mechanism of action: ADP activates platelets via two purinergic surface receptors, P2Y1 and P2Y12. P2Y1 receptors increase intracellular Ca2+ and initiate platelet shape change. Activation of P2Y12 receptors inhibits adenylyl cyclase, and reduces generation of the intracellular cyclic nucleotides that inhibit activation of GPIIb/IIIa receptors.
ADP receptor antagonists inhibit platelet aggregation by binding selectively to P2Y12 receptors (Fig. 11.2). Inhibition of P2Y12 receptors also reduces the production of thromboxane A2 by the platelet. Clopidogrel and prasugrel are irreversible receptor inhibitors, while ticagrelor binds to a different site on the receptor and produces reversible inhibition. There is considerable inter-individual variability in the degree of platelet inhibition by clopidogrel, and it has a slow onset of action (about 5 days for full effect) without a loading dose. Both prasugrel and ticagrelor are more predictable inhibitors of platelet activation than clopidogrel and have a more rapid onset of action.
Pharmacokinetics: Clopidogrel is a prodrug. It is well absorbed from the gut, and is activated by metabolism in the liver to a derivative that has a half-life of 7 h. Prasugrel is also a prodrug that is well absorbed from the gut and metabolised rapidly in the liver to an active metabolite which has a long half-life of 8 days. Ticagrelor is active as the parent drug, and also has an active metabolite. The offset of action of ticagrelor over 3 days is much slower than would be predicted from the short half-life.
Glycoprotein IIb/IIIa receptor antagonists
Mechanism of action: Abciximab is a murine/human chimaeric monoclonal antibody to the GPIIb/IIIa receptors with the Fc fragment removed to prevent clearance of antibody-bound platelets from the circulation. Abciximab binds irreversibly to the GPIIb/IIIa receptors and blocks the binding of fibrinogen (Fig. 11.2). Abciximab can reduce platelet aggregation by more than 90%.
Eptifibatide is a synthetic peptide that binds reversibly to and blocks the GPIIb/IIIa receptor.
Pharmacokinetics: All GPIIb/IIIa antagonists are given intravenously, usually as an initial bolus to achieve rapid inhibition of platelets followed by continuous infusion. The duration of receptor blockade with abciximab is longer than predicted from its very short half-life of 30 min due to slow dissociation from the receptor over several hours. After stopping abciximab, platelet aggregation largely recovers by 48 h as new platelets are synthesised.
Eptifibatide has a short half-life of about 2.5 h, and is eliminated by the kidney. Platelet aggregation recovers more rapidly after treatment than with abciximab, due to rapid dissociation of the drug from the receptor after a few seconds.
Epoprostenol
Mechanism of action: Epoprostenol (PGI2) increases platelet cAMP, which at low concentrations inhibits platelet aggregation and at higher concentrations reduces platelet adhesion. Epoprostenol is also a peripheral arterial vasodilator.
Pharmacokinetics: Epoprostenol is given by intravenous infusion. Unlike most other prostaglandins it is not significantly metabolised in the lung, as it is rapidly metabolised by hydrolysis in plasma and peripheral tissues, giving a very short half-life of about 3 min.
Clinical uses of antiplatelet drugs
Aspirin has often been used as the sole antiplatelet drug in a variety of clinical settings. However, there are some situations where clopidogrel is more effective, or where combinations of aspirin and an ADP receptor antagonist give better outcomes than aspirin alone. The suppression of thromboxane A2 production by ADP receptor antagonists has called into question whether the concurrent use of aspirin is necessary to achieve optimal clinical outcomes, but this issue is unresolved. A combination of antiplatelet drugs inevitably carries a greater risk of bleeding than a single agent. Main uses of antiplatelet drugs are listed here.
 Secondary prevention of embolic stroke and transient cerebral ischaemic attacks (aspirin, clopidogrel, dipyridamole). Clopidogrel alone is more effective than aspirin alone for the secondary prevention of stroke, while the combination has no further advantage despite a higher risk of bleeding. Dipyridamole combined with aspirin is better than aspirin alone for prevention of recurrent transient ischaemic attacks, and is equally effective as clopidogrel after stroke (Ch. 9).
Secondary prevention of embolic stroke and transient cerebral ischaemic attacks (aspirin, clopidogrel, dipyridamole). Clopidogrel alone is more effective than aspirin alone for the secondary prevention of stroke, while the combination has no further advantage despite a higher risk of bleeding. Dipyridamole combined with aspirin is better than aspirin alone for prevention of recurrent transient ischaemic attacks, and is equally effective as clopidogrel after stroke (Ch. 9). Secondary prevention after acute coronary syndrome (aspirin, clopidogrel, prasugrel, ticagrelor, eptifibitide, tirofiban). The combination of aspirin and clopidogrel is better than aspirin alone for reducing further vascular events after myocardial infarction. Prasugrel and ticagrelor may have advantages over clopidogrel in some situations (Ch. 5).
Secondary prevention after acute coronary syndrome (aspirin, clopidogrel, prasugrel, ticagrelor, eptifibitide, tirofiban). The combination of aspirin and clopidogrel is better than aspirin alone for reducing further vascular events after myocardial infarction. Prasugrel and ticagrelor may have advantages over clopidogrel in some situations (Ch. 5). Reduction of ischaemic complications produced by stent thrombosis following percutaneous coronary intervention (PCI) with stent insertion; these complications include non-fatal myocardial infarction, death and the need for emergency surgical revascularisation. Aspirin with clopidogrel or prasugrel are given for up to a year, often with abciximab or eptifibatide added at the time of the procedure when PCI is carried out following myocardial infarction.
Reduction of ischaemic complications produced by stent thrombosis following percutaneous coronary intervention (PCI) with stent insertion; these complications include non-fatal myocardial infarction, death and the need for emergency surgical revascularisation. Aspirin with clopidogrel or prasugrel are given for up to a year, often with abciximab or eptifibatide added at the time of the procedure when PCI is carried out following myocardial infarction. Secondary prevention of myocardial infarction in stable angina or peripheral vascular disease (aspirin, clopidogrel): either aspirin or clopidogrel alone is effective, and there is no evidence to support combination therapy (Chs 5 and 10).
Secondary prevention of myocardial infarction in stable angina or peripheral vascular disease (aspirin, clopidogrel): either aspirin or clopidogrel alone is effective, and there is no evidence to support combination therapy (Chs 5 and 10). Primary prevention of ischaemic heart disease (aspirin). This is a controversial area, with the potential for serious haemorrhage offsetting much of the potential benefit. Use of aspirin should be confined to people at very high risk of developing cardiovascular disease.
Primary prevention of ischaemic heart disease (aspirin). This is a controversial area, with the potential for serious haemorrhage offsetting much of the potential benefit. Use of aspirin should be confined to people at very high risk of developing cardiovascular disease. Anticoagulation in extracorporeal circulations; for example, cardiopulmonary bypass and renal haemodialysis (epoprostenol).
Anticoagulation in extracorporeal circulations; for example, cardiopulmonary bypass and renal haemodialysis (epoprostenol). Dipyridamole is used as a pharmacological stress for the coronary circulation to detect myocardial ischaemia in people who are unable to exercise. This is related to its ability to block the cellular uptake of adenosine. In the heart, adenosine acts on specific receptors in the small resistance coronary arteries to produce vasodilation. Dipyridamole can divert blood away from myocardium supplied by stenosed coronary arteries by preferentially dilating healthy vascular beds (vascular steal).
Dipyridamole is used as a pharmacological stress for the coronary circulation to detect myocardial ischaemia in people who are unable to exercise. This is related to its ability to block the cellular uptake of adenosine. In the heart, adenosine acts on specific receptors in the small resistance coronary arteries to produce vasodilation. Dipyridamole can divert blood away from myocardium supplied by stenosed coronary arteries by preferentially dilating healthy vascular beds (vascular steal).Anticoagulant drugs
Anticoagulation can be achieved with either injectable or oral drug therapy. Increasingly, oral anticoagulant therapy with newer agents is likely to supersede the long-established use of heparin followed by warfarin to initiate anticoagulation.
Injectable anticoagulants
Heparins are a family of highly sulphated acidic mucopolysaccharides (glycosaminoglycans) that are found in mast cells, basophils and endothelium. Heparins have a variable molecular weight of between 3000 and 30 000 Da according to the numbers of polysaccharide subunits.
Mechanism of action and effects: Heparin is available as an unfractionated preparation, or as low-molecular-weight heparins (LMWHs), which consist of the heparin subfractions that have molecular weights of less than 7000 Da.
Unfractionated heparin forms a complex with and alters the conformation of antithrombin III; this complex can then inactivate thrombin and factors IXa, Xa, XIa and XIIa (Fig. 11.3). LMWH interacts with antithrombin III in a different manner to unfractionated heparin, and the LMWH–antithrombin complexes have a more selective anticoagulant action, mainly inhibiting factor Xa (Fig. 11.3).
Additional actions of the heparins are as follows.
 Promotion of tissue factor pathway inhibitor (TFPI) release from the vascular wall contributes to the antithrombotic effects of heparin. TFPI inhibits formation of factor Xa.
Promotion of tissue factor pathway inhibitor (TFPI) release from the vascular wall contributes to the antithrombotic effects of heparin. TFPI inhibits formation of factor Xa.Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree



















