, Christopher VandenBussche2 and Syed A. Hoda3
(1)
CBL Path, Rye Brook, NY, USA
(2)
Department of Pathology, Johns Hopkins University Department of Pathology, Baltimore, MD, USA
(3)
New York Presbyterian Hospital Weill Cornell Medical College, New York, NY, USA
Introduction
Cytological screening for cervical carcinoma and its precursor lesions using the Papanicolaou smear test (Pap test) has been efficient in reducing its morbidity and mortality. Currently, cervical cancer is the seventh leading cause of cancer deaths in the United States. In 2016, about 12,340 new cases of invasive cervical cancer will be diagnosed, and about 4030 women will die from the disease. While the conventional Pap smear (CPS) has been successful in reducing the incidence and mortality rate from cervical cancer, it has limitations, particularly with respect to false-negative screening results. Several properties of CPS lead to high false-negative results such as: In CPS, the collected cell sample is smeared on the slides which often results in thick cellular areas making the finding of an abnormality difficult. Other background elements such as blood, inflammatory cells, and necrosis may also obscure diagnostic cells; and artifacts such as air-drying and crush effect may further compromise the specimen. The liquid-based preparations (LBPs) have revolutionized the Pap test by allowing standardization of cervical specimen collection, processing, and screening. LBP has shown a significant improvement in the detection of precursor lesions of cervical neoplasia. In LBP, the cells are rinsed into a liquid preservative collection medium [CytoLyt for ThinPrep (TP) and BD® SurePath (SP) preservative for SP specimens]. This immediate wet fixation of specimen ensures good preservation and reduces the time for air-drying artifact to be introduced. Specimen processing is automated and, thus, standardized and uniform. TP specimens are processed using TP2000 or TP5000 processors, and SP specimens are processed using PrepMate or Totalys System. Residual specimens left in collection media can be used for ancillary testing of human papillomavirus (HPV), chlamydia, and gonorrhea. Despite the differences in preparatory techniques, the two cytological preparations using LBP (TP and SP) are largely similar; however, subtle differences exist. The Bethesda System (TBS) for Reporting Cervical Cytology, updated in 2014, provides criteria for Pap test diagnosis.
Alterations in General Features in LBP
The main alterations occur in background, architecture, and cellular morphology. These alterations are probably due to technical reasons (Please see Chap. 1).
The 2014 Bethesda System for Reporting Cervical Cytology
An update to TBS was released in 2014. This update allowed improved clarifications to the guidelines, as well as the addition of images [1]. In contrast to the previous guidelines, the 2014 guidelines recommend that the “other” category of benign-appearing endometrial cells be reported in women age of 45 or greater (previously, the age cutoff was more than or equal to 40). The guidelines also address the use of a “low-grade squamous intraepithelial lesion/cannot exclude high-grade squamous intraepithelial lesion” (LSIL-H) diagnostic category; this category has not been accepted by TBS, and the 2014 guidelines recommend against its use.
American Society for Colposcopy and Cervical Pathology (ASCCP) Interim Guidelines for Primary High-Risk HPV (hrHPV) Testing
In 2011, the American Society for Colposcopy and Cervical Pathology (ASCCP) released updated consensus guidelines for the management of abnormal Pap tests [3]. Following approval of HPV testing as a primary screening test for cervical dysplasia by the Food and Drug Administration (FDA) in 2014, the ASCCP released interim guidelines for primary HPV testing. While cytology alone and cotesting are the only screening options specifically recommended in major guidelines, primary high-risk HPV (hrHPV) screening can be considered as an alternative. While data is still limited, the interim guidelines suggest that hrHPV-positive women should have HPV genotyping performed, with HPV 16/18-positive women being triaged to colposcopy and women positive for the other 12 hrHPV genotypes triaged to cytology. With the latter triage, a diagnosis of ASC-US or higher on the Pap test results should be sent to colposcopy. Primary hrHPV screening should not be performed in women below the age of 25.
HPV Vaccination
Three HPV vaccines have been approved by the FDA: Gardasil, Gardasil 9, and Cervarix. All three vaccines protect against HPV types 16 and 18, which cause approximately 2/3 of all cervical cancers. Gardasil also prevents HPV types 6 and 11, which cause the majority of genital condylomas, and Gardasil 9 protects against five additional high-risk HPV types (31, 33, 45, 52, and 58). Females and males between the ages 9 and 26 are eligible for Gardasil, except Gardasil 9 is only approved for males between the ages 9 and 15. Cervarix is approved for use in females between 9 and 25 years old. Studies have shown protection to last for at least 8–9 years. However, given the relatively recent use of these vaccines, their long-term effects remain unknown; therefore, it is difficult to predict how widespread HPV vaccination will impact HPV prevalence and future screening guidelines for cervical cancer and dysplasia.
Immunocytochemistry (ICC) on LBP
Several studies have reported on the utility of ICC in LBP. Immunostaining on LBP shows equal or greater intensity and proper distribution of staining compared to CPS. Good results have been reported for epithelial, lymphoid, neuroendocrine, sarcoma, melanoma, molecular, and prognostic markers. However, ICC on cell block (CB) gives superior results compared to LBP.
Assimilation of HPV oncogenes E6 and E7 into the host DNA promotes upregulation of cyclin-dependent kinase inhibitor (CDKI) p16 (INK4A). The latter is detectable by monoclonal antibody in the developing cervical cancer cells. p16 immunostaining has been successfully applied to LBP for both squamous and glandular lesions. An association has been shown between strong p16INK4A immunostaining of atypical squamous/glandular cells in smears and the presence of a significant lesion in the cervix. This antibody is not expressed in normal glandular cells. p63 stains basal cells and may be a diagnostic pitfall in atrophic Pap tests.
Automation
One benefit of LBP is the automation used for both specimen preparation as well as digital screening of specimens. This is important since Pap tests remain the largest specimen type in most cytopathology laboratories, and automation decreases laboratory technician, cytotechnologist, and cytopathologist workload, which in turn allows for increased productivity. For instance, the ThinPrep TP5000 processor allows continuous, hands-free processing of 20 Pap and non-Gyn specimens. Coupled with an autoloader, the system can allow for up to 8 h of hands-free technician time. Multiple automated systems for the digital screening of Pap tests exist, such as the ThinPrep Imaging System (TIS, Hologic Corp., Marlborough, MA) for use on TP and BD FocalPoint Slide Profiler and BD FocalPoint GS™ Imaging System (BD Diagnostic, Burlington, NC) for use in SP and CPS. These systems allow for Pap tests to be digitally screened.
Conclusion
In conclusion, LBP are of great value for the processing of gynecologic specimens (Figs. 2.1–2.28). Most diagnostic criteria utilized on CPS are also applicable to LBP. The seitivity and specificity of LBP are either comparable or superior to CPS in detecting abnormalities. The unsatisfactory rate is lower in comparison o CPS. LBP are less time-consuming to screen and easier to interpret, as the cells are limited to a smaller area on a cleaner background with excellent cellular preservation. The use of LBP has the potential to decrease turnaround time and increase the number of specimens being tested in a laboratory without corresponding increase in technical staff, a quality which also makes LBP more cost-effective. LBP requires familiarity with alterations in background, extracellular elements, architecture, and cell morphology. Experience (and in some situations, modification to diagnostic criteria previously utilized for CPS) may be required.






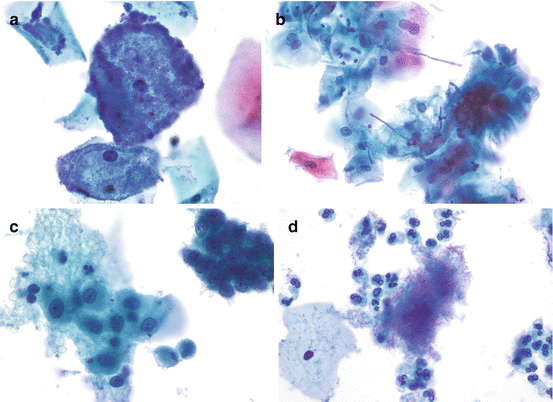



Fig. 2.1
Benign squamous cells. (a, b) Superficial cells are flat with abundant, typically eosinophilic cytoplasm and pyknotic nuclei. Intermediate cells are also flattened with abundant basophilic or eosinophilic cytoplasm and vesicular nuclei. Parabasal cells are round and have basophilic, dense cytoplasm; the nucleus is larger and typically round or oval in shape; and small nucleoli are often present (a, TP; b, SP)

Fig. 2.2
Atrophy. (a) Parabasal cells with mostly bland nuclei and some with eosinophilic cytoplasm are seen. Interspersed degenerated cells with pyknotic nuclei are also present. Background shows clumped basophilic granular debris and inflammation (SP). (b) Atrophy shows a cluster of parabasal and basal cells. Parabasal cells, seen toward the periphery, are rounder with dense well-defined cytoplasm, round slightly dark, uniform nuclei and small nucleoli. Nuclear to cytoplasmic ratio is low. Basal cells have oval nuclei and less cytoplasm with a relatively high nuclear to cytoplasmic ratio. Cells with eosinophilic cytoplasm are lacking (TP). An atrophic picture can result from many different clinical etiologies, most of which are associated with decreased estrogen (e.g., postmenopause or postpartum). Cells with high nuclear to cytoplasmic ratios may cause suspicion for high-grade squamous intraepithelial lesion (HSIL). In atrophic vaginitis, the background shows clumps of granular inflammatory debris, similar to the diathesis seen in squamous cell carcinoma and distinguished from it only by the absence of malignant cells in atrophy. Inflammation does not obscure cell detail in LBP

Fig. 2.3
Navicular cells. Navicular cells are intermediate squamous cells containing glycogen. In LBP, glycogen may be lost during processing and appear as an equivocal cytoplasmic halo with a thick rim, which if visualized carefully appears to be a cytoplasmic fold. The nuclei are uniform, small, and vesicular. Low-grade squamous intraepithelial lesion (LSIL) may be mistaken for navicular cells and vice versa. However, normal intermediate cell nuclei are helpful in avoiding overinterpretation (TP)

Fig. 2.4
Repair. In contrast to conventional smears, cells with repair changes appear as cohesive monolayer sheets of cells with less “streaming” effect and rounded, instead of frayed, cell borders. The staining of cells may also be more uniform with less polychromasia. Cells may be bi- or multinucleated with enlarged, round to oval, uniform, and regular nuclei and evenly dispersed pale chromatin. Macronucleoli are the most significant feature; cytoplasm may be vacuolated with intracytoplasmic neutrophils. Nuclear to cytoplasmic ratio is low and background may show inflammation (TP). The differential diagnosis is similar to conventional smear and includes invasive squamous cell carcinoma. In the College of American Pathologists (CAP) Interlaboratory Comparison Program in Gynecologic Cytology, TP slides with a reference diagnosis of reparative change had a lower false-positive and discordance rate and a higher exact match error rate than CPS

Fig. 2.5
Benign glandular endocervical cells. (a, b) Endocervical cells are present and evenly spaced in a honeycomb sheet with retained polarity and no cellular overlap. The cytoplasm is mucinous, and nuclei are round and uniform with pale chromatin, and chromocenters with occasional protrusion (“nippling”). Benign endocervical cells appear similar in conventional smears and LBP. However, individual cells may be tall and columnar, a feature that is more pronounced in SP. In SP, nuclear contour is regular. The cytoplasm is often dense with small vacuoles and distinct cell borders, and the nuclear to cytoplasmic ratio may be slightly increased (a, TP; b, SP)

Fig. 2.6
Benign endometrial cells. (a, b) Endometrial cells are arranged as a three-dimensional group with scalloped borders. The nuclei are round and small (equal to intermediate cell nuclei) and possess small nucleoli or chromocenters. The cytoplasm is vacuolated (a, TP; b, SP). Exodus as seen in (a) appears as a double-contoured round to oval cluster of epithelial cells surrounding a dense core of stromal cells. Apoptosis may be present. (c) Superficial stromal cells are round cells with round pale nuclei and foamy cytoplasm. These cells tend to stay together as a loosely cohesive group. (d) Deep stromal cells are elongated with elongated, spindled, and dark nuclei and scant bipolar cytoplasm. In CPS, the cytoplasm is not usually evident (c, d, TP). The differential diagnosis of deep stromal cell includes HSIL. Immunostain for p16 may be helpful as it would be positive in HSIL, while CD10 would be positive in deep stromal cells

Fig. 2.7
Bacterial microorganisms. (a) “Shift in vaginal flora” or bacterial vaginosis. The pathognomonic “clue cells” are intermediate squamous cells heavily covered with bacteria, giving a “shag carpet” appearance. The background is usually clean with clumps or loose clusters of organisms and is devoid of inflammatory cells (TP). (b) Candida sp. pseudohyphae appear as long basophilic to pinkish structures skewering the superficial squamous cells in a “shish kebab” appearance; yeast forms may also be present (TP). (c) Trichomonas vaginalis. The organisms appear small and pear shaped (occasionally “kite shaped”) with an eccentric single almond-shaped basophilic nucleus, cherry-red or basophilic granules, and one or more flagella. The organisms may overlie squamous cells, particularly in SP. Although the clean background of LBP makes identification of Trichomonas less laborious, the smaller-sized trophozoites may be eliminated during processing. Degenerating parabasal and endocervical cell cytoplasmic fragments may mimic Trichomonas. Squamous cells show reactive changes (TP). (d) Actinomyces. The organism appears as gray-blue to black-staining small islands of dense amorphous material. Closer examination reveals tangled clusters and haphazardly arranged filamentous bacterial colonies which branch at acute angles and often contain blunt ends. Several loose organisms are also seen admixed with inflammatory cells (TP)

Fig. 2.8
Herpes simplex virus. The morphology is similar to conventional smear except that the virus is easier to detect in LBP due to the clean background. Note the multinucleation and molding of ground-glass nuclei, the latter consisting of the actual viral particles. Cytoplasmic inclusions may also be seen (TP)

Fig. 2.9
Atypical squamous cells of undetermined significance (ASC-US). (a, b) Mature squamous cells, in TP, show nuclear features of slight enlargement, subtle membrane irregularities, and chromatin changes. In both TP and SP, cytoplasm shows suggestion of koilocytotic halos (a, TP; b, SP). The cytological criteria for ASC-US in conventional smears and LBP are similar. As a result of the improved ability to discern benign mimickers of ASC-US, LBP has shown a decrease in the interpretation of ASC-US, and this diagnosis is associated with an increased likelihood of representing a significant lesion. In LBP, ASC-US cells are more likely to be dispersed




