Chapter 25 Cyanogenetic glycosides, glucosinolate compounds, cysteine derivatives and miscellaneous glycosides
CYANOGENETIC GLYCOSIDES
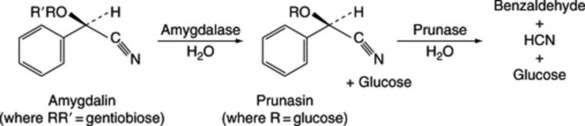
Many of these glucosides, but not all, are derived from the nitrile of mandelic acid. Although they contain nitrogen their structure is that of O-and not N-glycosides. The sugar portion of the molecule may be a monosaccharide or a disaccharide such as gentiobiose or vicianose. If a disaccharide, enzymes present in the plant may bring about hydrolysis in two stages, as in the case of amygdalin (amygdaloside), Fig. 25.1.
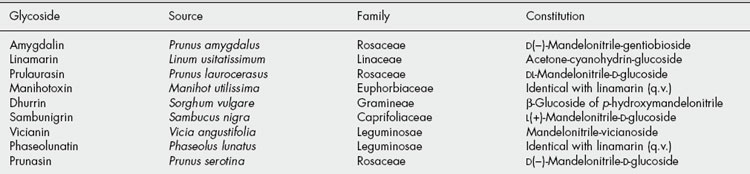
Table 25.1 gives some well-known cyanogenetic glycosides isolated from various sources between 1830 and 1907.
Biogenesis
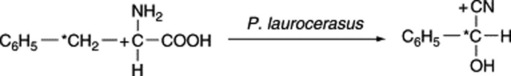
The aglycones of cyanogenetic glycosides are derived solely from nitrogen intermediates. The biosynthesis of prulaurasin (DL-mandelonitrile glucoside) has been studied in the leaves of Prunus laurocerasus. Phenyl[3-14C]alanine, phenyl[2-14C]alanine and phenyl[1-14C]alanine were fed to the leaves and the hydrolytic products of the isolated glycosides were examined. The three labelled precursors gave, respectively, active benzaldehyde and inactive hydrocyanic acid; inactive benzaldehyde and active hydrocyanic acid; and inactive benzaldehyde and active hydrocyanic acid; and inactive hydrolytic products consistent with the following incorporation:
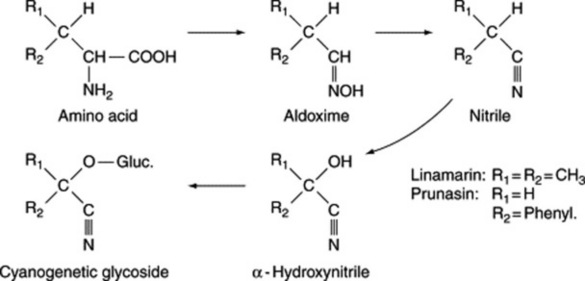
Similarly, phenyl[2-14C]alanine fed to P. amygdalus gives amygdalin with most activity in the carbon atom of the nitrile. Experiments with doubly labelled amino acids have shown that the nitrile nitrogen of the cyanogen is derived from the nitrogen atom of the amino acid. Similar results have been obtained with dhurrin isolated from sorghum seedlings fed with labelled tyrosine. More recent work has sought to determine the nature of the intermediates involved in the above conversions and, for prunasin and linamarin, the participation of oximes and nitriles has been demonstrated (Fig. 25.2).
Wild cherry bark
History
The drug was introduced into American medicine about 1787 and appeared in the USP in 1820. It first attracted notice in Britain about 1863.
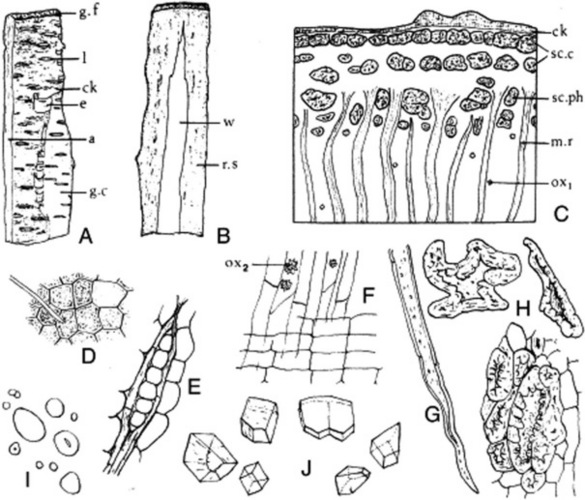
Macroscopical characters
The drug usually occurs in curved or channelled pieces up to 10 cm long, 5 cm wide and 0.3–4.0 mm thick (Fig. 25.3). Much larger pieces of trunk bark, up to 8 mm thick, may be found but the BP (1980) maximum thickness is 4.0 mm and is known commercially as ‘Thin Natural Wild Cherry Bark’. The branch bark, if unrossed, is covered with a thin, glossy, easily exfoliating, reddish-brown to brownish-black cork, which bears very conspicuous whitish lenticels. In the rossed bark pale buff-coloured lenticel scars are seen and the outer surface is somewhat rough, some of the cortex having been removed and the phloem exposed. The inner surface is reddish-brown and has a striated and reticulately furrowed appearance, which is caused by the distribution of the phloem and medullary rays. Patches of wood sometimes adhere to the inner surface. The drug breaks with a short, granular fracture. When slightly moist it has an odour of benzaldehyde. Taste is astringent and bitter.
< div class='tao-gold-member'>
Stay updated, free articles. Join our Telegram channel

Full access? Get Clinical Tree