Giant Prosthesis for Reinforcement of the Visceral Sac in the Repair of Groin and Incisional Hernias
René E. Stoppa
Simply suturing—even without tension—the deep musculofascial layer of the groin is not sufficient to repair a hernia with a high risk of recurrence. For almost 30 years, instead of making useless efforts to repair an irreparable inguinal wall, I have reinforced the lower part of the visceral sac so that the herniation cannot persist. This part of the sac is broadly wrapped in a giant bilateral piece of nonabsorbable mesh and placed in a position underlying the wall through a midline subumbilical incision. The device is known as a giant prosthesis for reinforcement of the visceral sac, or GPRVS. Providing almost absolute proof against recurrence and a major expression of prosthetic repair of groin hernias, this method has been said to also be a model for some new laparoscopic trends, which it certainly is not. Principles similar to those of the GPRVS can be usefully applied to the difficult repair of large incisional hernias (LIHs), which is considered briefly at the end of this chapter.
The operation is most often used for re-recurrent hernias, multiple herniations associated with different types of groin hernias, and groin hernias associated with one or multiple lower-incisional hernias. Reasonable indications are complicated hernias, such as sliding hernias and huge inguinoscrotal hernias. The operation is also indicated when the patient has a condition such as obesity, advanced age, or cirrhotic ascites that presents greater risk than usual. The most delicate point to discuss is the use of this technique in primary repair in men older than 50 years whose activities require physical effort. Under these circumstances, the risk of recurrent herniation must be balanced against the greater scale of the giant prosthetic repair and the impossibility of using local anesthesia or 1-day surgical care. In some circumstances, one may waver between a traditional repair and the radicalness of a prosthesis because “he who can do the more can do the less.”
As with every procedure, contraindications and limits exist for GPRVS. An absolute contraindication is the risk of sepsis related to recent dermatosis in the region or associated surgery with the possibility of sepsis. Relative contraindications are a midline scar from a previous operation, a history of iliocaval vein thrombosis, and the impossibility of using general or spinal anesthesia; in the last of these situations, a Wantz operation—a unilateral, half-cut GPRVS—can be performed under local anesthesia.
Principles: the Musculopectineal or Myopectineal Orifice
For ethical, professional, and economic reasons, these are the days of hernial surgery without recurrences. Prosthetic repair has arrived, making possible the treatment of damaged inguinal walls.
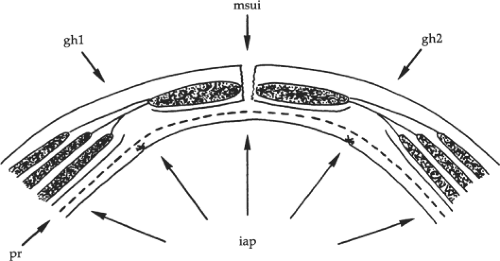
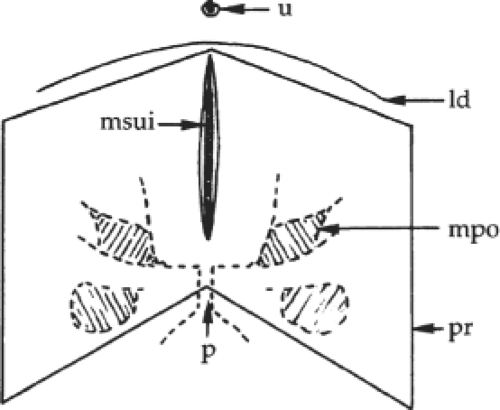
Following Fruchaud, my colleagues and I propose to unify the anatomic concept of groin hernias because all of them cross the groin structures through the musculopectineal orifice (Fig. 1), wherever they emerge in the inguinal or femoral regions. Within the musculopectineal orifice, the drum-like transversalis fascia is a taut layer that resists intra-abdominal pressure (Fig. 2). A mesh prosthesis can reinforce—or, when the transversalis fascia is deficient, replace—this layer. Behind the endoabdominal fascia, the cleavage spaces of Retzius and Bogros spread widely medially and laterally on both sides. These spaces are a useful surgical route to reach deep hernial defects. They are also a perfect site for placement of the giant prosthesis used in our method, in a position dorsal (posterior) to most of the abdominal wall.
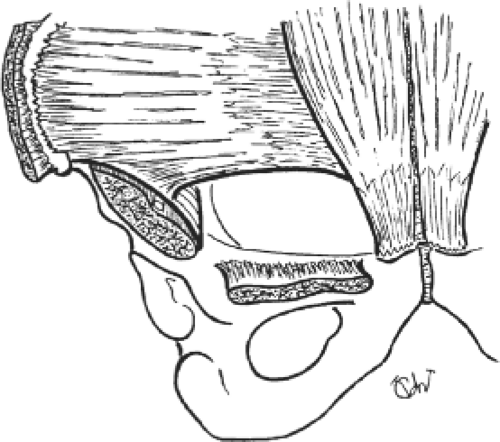 Fig. 1. The musculopectineal orifice described by Fruchaud. The weak area of the inguinal wall is tightened against the intra-abdominal pressure by the single drum-like transversalis fascia. |
Fascial repair is valuable in repairing congenital groin hernias, which are caused by permanent patency of the peritoneovaginal channel. In addition, other hernias—inguinal or femoral—related to fascial weakness may logically necessitate the use of a prosthesis for sufficient coverage. Re-recurrent hernias are most often best corrected with a prosthesis.
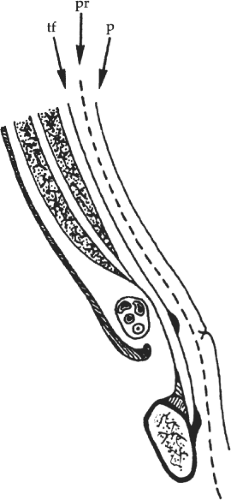 Fig. 2. Sagittal section of the wall of the groin, showing the deep position of the transversalis fascia (tf). p, peritoneum; pr, prosthesis. |
Among the posterior approaches to groin hernias, the preperitoneal route offers the most interesting resources to the surgeon. Proposed by Cheatle and popularized by Harkins, this approach has been aptly recounted in the work of Nyhus. We have used the preperitoneal subumbilical
midline approach since 1965. The advantages are most striking in the repair of re-recurrent hernias. The approach allows easy separation of the retroperitoneal spaces without bleeding, and clear and direct exposure of the musculopectineal and hernial orifices without worsening existing defects in inguinal structures and without injuring superficial nerves, cord elements, or femoral vessels. A feature of the abdominal midline approach is that it allows one to insert a large piece of synthetic mesh that covers the dimensions and depth of the defect. Because of the risk of incisional hernia induced by the midline incision, we use only bilateral giant prostheses that both tighten the weak inguinal area bilaterally and protect the medial ridge. I use polyester Dacron mesh for the prosthesis. It is well tolerated but induces sufficient fibroplastic reaction for rapid fixation that is resistant, supple, lightly adherent to the tissues (which helps its instant stability), and inexpensive (Fig. 3). Polypropylene meshes are less convenient than Dacron because they are less supple.
midline approach since 1965. The advantages are most striking in the repair of re-recurrent hernias. The approach allows easy separation of the retroperitoneal spaces without bleeding, and clear and direct exposure of the musculopectineal and hernial orifices without worsening existing defects in inguinal structures and without injuring superficial nerves, cord elements, or femoral vessels. A feature of the abdominal midline approach is that it allows one to insert a large piece of synthetic mesh that covers the dimensions and depth of the defect. Because of the risk of incisional hernia induced by the midline incision, we use only bilateral giant prostheses that both tighten the weak inguinal area bilaterally and protect the medial ridge. I use polyester Dacron mesh for the prosthesis. It is well tolerated but induces sufficient fibroplastic reaction for rapid fixation that is resistant, supple, lightly adherent to the tissues (which helps its instant stability), and inexpensive (Fig. 3). Polypropylene meshes are less convenient than Dacron because they are less supple.
 Fig. 3. A piece of the polyester Dacron mesh used for the prosthesis—supple, sticky, available in very large dimensions, and cheap. |
Because of its tissue tolerance, Dacron mesh can be used for giant prostheses, widely interposed between the visceral sac and the endoabdominal fascia, instantly and definitely reinforcing the weak inguinal areas. Pascal’s hydrostatic principle explains that the intra-abdominal pressure fixes the prosthesis in position, secondarily invested by the scar connective tissue (Fig. 4). The two revolutionary foundations of our method are to render the visceral sac inextensible and to use the force that usually causes herniation (intra-abdominal pressure) to prevent its recurrence. This system is similar to the seal between an inner tube and a tire. The larger the prosthesis, the more efficient is the repair. Neither fixation of the prosthesis nor direct repair of the hernial orifices is necessary because of the efficiency of the giant prosthesis.
Preoperative Preparation
Because of the consequences of sepsis, careful local disinfection is mandatory. Patients with large inguinoscrotal hernias, the contents of which have lost their place in the abdomen, and patients suffering ventilatory insufficiency may need progressive pneumoperitoneum. The operation for the insertion of the giant prosthesis requires general, spinal, or epidural anesthesia. We advise surgeons against the use of local anesthesia, but point out that Wantz uses it in a unilateral half-cut GPRVS.
The following equipment should be present: mounted peanut swabs for the retroperitoneal separation; straight valve retractors for retraction of the abdominal wall; Ombredanne forceps or snares for handling the spermatic cord; a sterile ruler; straight scissors, and eight curved Kelly forceps for the measurement, cutting, and no-touch handling of the prosthesis; small suction drains; and slowly absorbing synthetic sutures.
Operation for A Typical Bilateral Hernia in A Male Patient
Midline Preperitoneal Approach
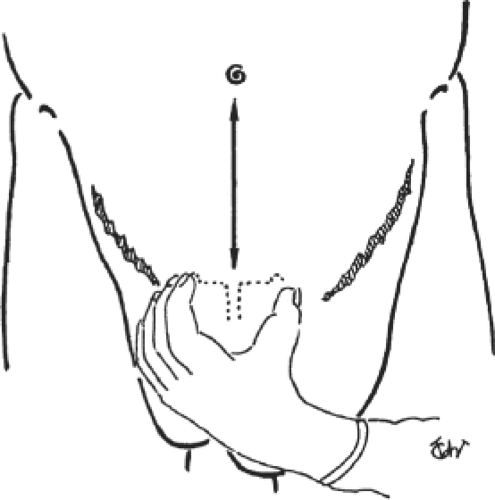
The patient lies in a slight Trendelenburg position. The surgeon and nurse stand on one side and the assistant on the other. After antiseptic preparation of the skin, an adhesive plastic shield is used to protect the incision from skin contamination. The abdominal wall is incised in the midline (Fig. 5). The umbilicoprevesical fascia is cut along its entire length with Mayo scissors.
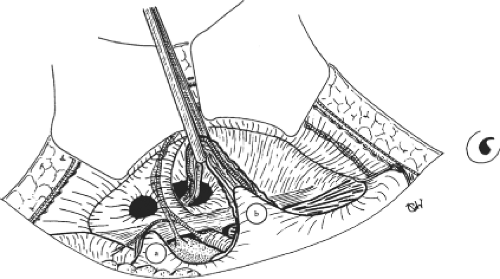
The preperitoneal and prevesical cleavage is begun inferiorly and medially in the space of Retzius with a mounted swab. Dissection progresses laterally under the rectus abdominis muscle on the side opposite the surgeon and posteriorly to the inferior epigastric vessels. Cleavage continues downward, anterior (ventral) to the bladder, to the prostatic fossa, and behind the iliopubic ramus in the space of Bogros. The pedicle of the hernia is isolated on the side opposite the surgeon, either distinct from the spermatic cord (in direct inguinal or femoral hernias) or connected to it (in indirect inguinal hernias) (Fig. 6). The iliopsoas muscle
and external iliac vessels within their sheath are exposed. This dissection does not require delicate pressure, even in re-recurrent hernias, and is not problematic for the surgeon regardless of level of experience. The hernial sacs are treated differently according to the degree of adhesion to the neck of the myopectineal orifice. In instances of primary treatment of moderate-sized hernias, continuous moderate traction followed by resection or turning inside out can be sufficient. Conversely, a strong adherent sac of a multirecurrent hernia with a large neck sometimes needs to be freed by scalpel or scissors, with a finger introduced through an opening in the peritoneal infundibulum to assist the dissection.
and external iliac vessels within their sheath are exposed. This dissection does not require delicate pressure, even in re-recurrent hernias, and is not problematic for the surgeon regardless of level of experience. The hernial sacs are treated differently according to the degree of adhesion to the neck of the myopectineal orifice. In instances of primary treatment of moderate-sized hernias, continuous moderate traction followed by resection or turning inside out can be sufficient. Conversely, a strong adherent sac of a multirecurrent hernia with a large neck sometimes needs to be freed by scalpel or scissors, with a finger introduced through an opening in the peritoneal infundibulum to assist the dissection.
After the sac has been treated, the preperitoneal cleavage is continued easily and quickly below the external iliac vessels and laterally along the psoas muscle. It is of no use to continue this work upward beyond the line of Douglas, where the peritoneum is adherent and prone to tearing. The cleavage of the spaces of Retzius and Bogros is done quickly and easily, with a single, flat retractor placed under the abdominal wall and the surgeon’s left hand displacing the peritoneum.
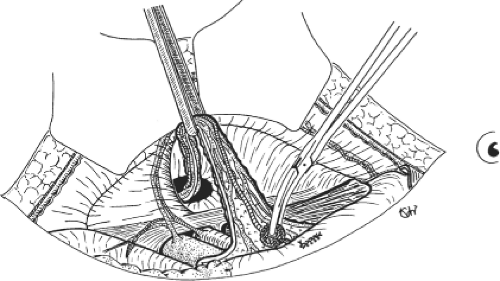
The parietalization of the components of the spermatic cord greatly simplifies the placement of the giant prosthesis so that opening the mesh to allow passage of the spermatic cord is not required. For this purpose, the spermatic cord is retracted with a snare so that scissors easily separate the funicular elements from the peritoneal envelope. Beginning at the retroperitoneal convergence of these structures, freeing is performed with mounted swabs. At this stage, one can see an areolar sheath of triangular shape—which is the retroparietal segment of the funicular sheath—that is carefully preserved; the medial margin contains the ductus deferens and the lateral margin contains the spermatic vessels (Fig. 7). When the funicular elements are relaxed, they appear parietalized, and no pedicle can be seen crossing the preperitoneal and prevesical cleavage spaces.
Now the surgeon changes sides to achieve the retroperitoneal cleavage on the opposite side, which proceeds as described previously.
Placement of the Giant Prosthesis
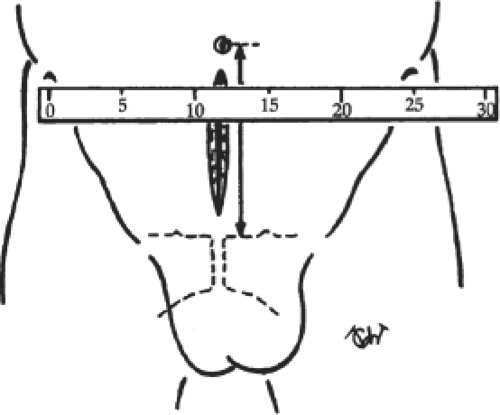
The prosthesis should be measured directly on the patient to allow the implantation of the largest piece possible. The correct transverse dimension of the prosthesis is equal to the distance between the right and left anterosuperior iliac spines, minus 2 cm, and the correct vertical dimension is equal to the distance between the umbilicus and the pubis (Fig. 8). The mean dimensions are 26 cm transversely and 15 cm vertically. The prosthesis is then cut into a chevron shape according to the patient’s measurements. A no-touch technique is mandatory. The
chevron shape allows the prosthesis to run beyond the musculopectineal orifices and to correspond to the line of Douglas (Fig. 9). Before being inserted, the mesh is briefly soaked in povidone–iodine solution.
chevron shape allows the prosthesis to run beyond the musculopectineal orifices and to correspond to the line of Douglas (Fig. 9). Before being inserted, the mesh is briefly soaked in povidone–iodine solution.
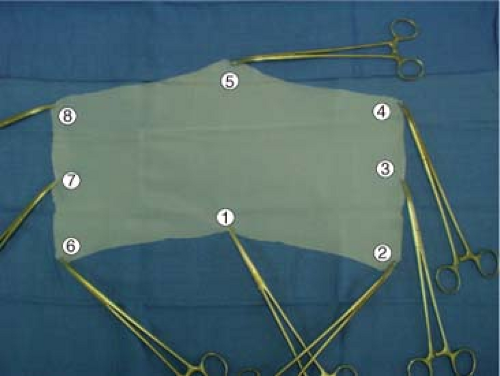 Fig. 10. Photograph of the chevron-shaped prosthesis grasped by eight long Kelly forceps for their no-touch handling and easy correct placement. The numbers 1 to 8 indicate the order of placement of the clamps, beginning on the right side of the patient.
Stay updated, free articles. Join our Telegram channel
Full access? Get Clinical Tree
 Get Clinical Tree app for offline access
Get Clinical Tree app for offline access

|