Chapter 12 Genitourinary System
Kidney – Structure and Function
The kidney has several functions:
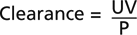 where:
where:Chronic Kidney Disease can be classified as follows:
| CKD | Stage | eGFR/mL/min/1.73m2 |
|---|---|---|
| 1 | with an abnormality* | >90 |
| 2 | with an abnormality* | 60–89 |
| 3 | 30–59 | |
| 4 | 15–29 | |
| 5 | <15 |
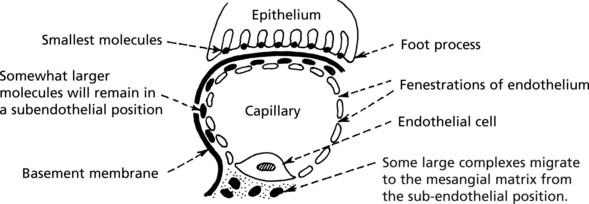
Glomerular Structure and Function
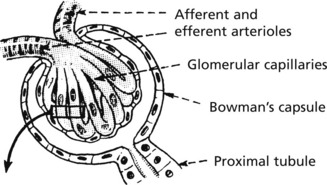
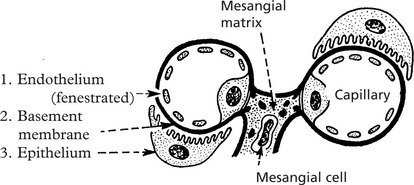
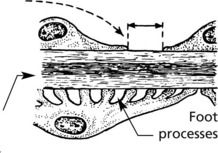
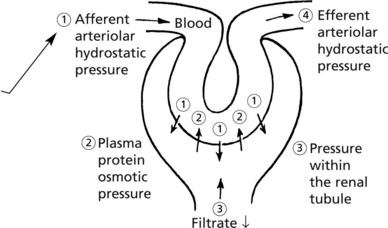
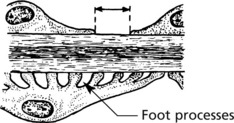
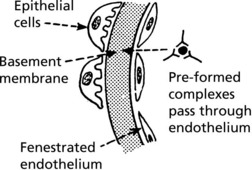
The glomerulus, of which there are over 600000 in the adult, consists of an invagination of a capillary network, derived from the afferent arteriole, into Bowman’s capsule – the beginning of the proximal tubule.
The glomerulus is an efficient filter due to the large surface area of the glomerular capillaries.
Glomerular Diseases
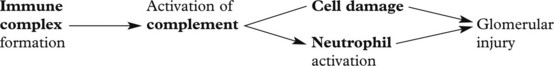
The mechanisms underlying these changes are as follows:
These lead to 4 main clinical syndromes:
The main forms of glomerular disease are described in the following pages.
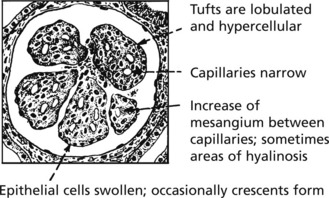
Acute Diffuse Proliferative Glomerulonephritis
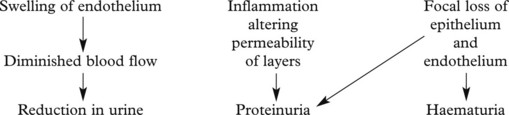
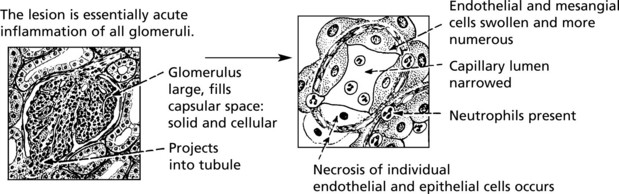
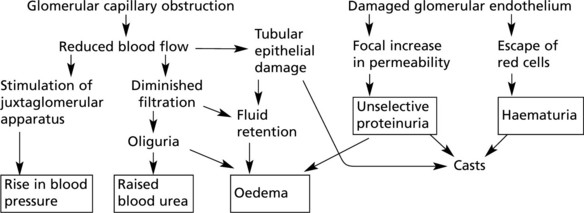
This disease classically follows 2–3 weeks after an infection – usually pharyngitis due to Group A haemolytic streptococci. It is commonest in children and young adults who develop the NEPHRITIC SYNDROME: oliguria, proteinuria, haematuria (urine is smoky and dark), moderate hypertension and facial (periorbital) oedema. This disease is now uncommon in fully developed countries.
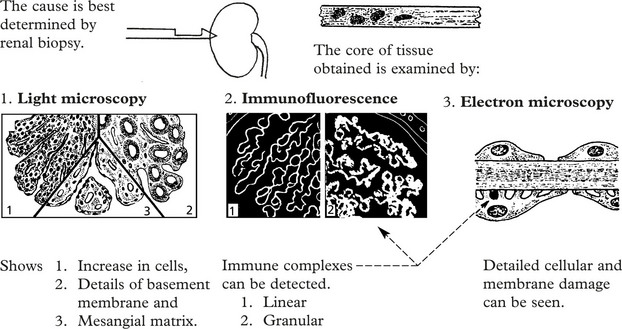
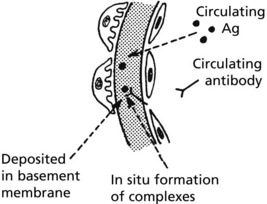
Immune complexes are identified by:
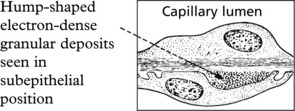
The clinical findings can be correlated with pathology as follows:
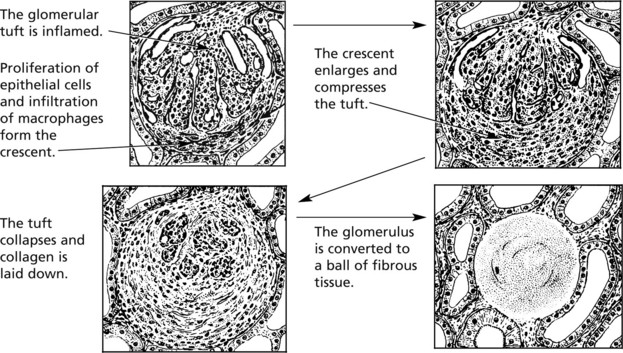
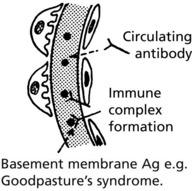
Crescentic (Rapidly Progressive) Glomerulonephritis
Without treatment, this disease progresses to end-stage renal failure in weeks or months. The histological hallmark is crescent formation in >50% of glomeruli.
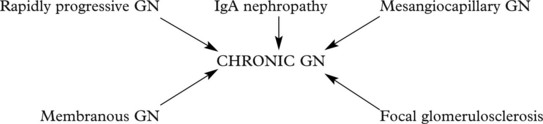
Many types of glomerulonephritis can progress to crescentic GN. Examples include:
IF shows linear deposits of IgG and C3 in the capillary basement membranes
Prognosis – Without treatment, most patients die within 6 months.
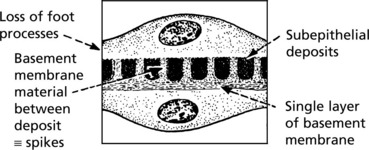
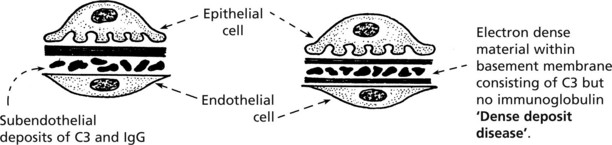
Membranous Glomerulonephritis
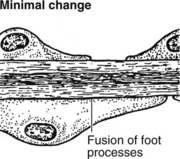
This accounts for around 30% of cases of the nephrotic syndrome in adults; some patients present with asymptomatic proteinuria.
Aetiology
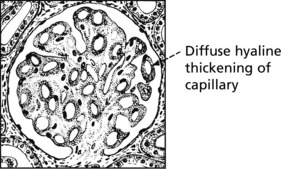
Pathology – There is generalised thickening of capillary basement membrane.
Specific silver staining of the basement membrane shows a typical pattern.
Immunofluorescence reveals deposition of IgG in the capillary walls.
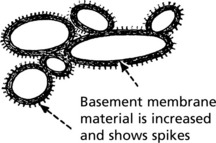
Electron microscope findings explain this appearance.
In the later stages there is a massive increase in basement membrane material.
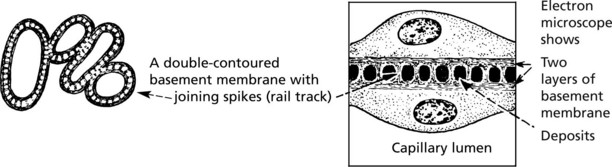
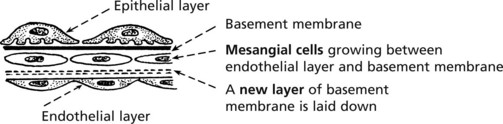
Mesangiocapillary (Membranoproliferative) Glomerulonephritis
In this form of glomerulonephritis there is an increase both in cells and mesangial matrix within glomeruli.
Silver staining of basement membrane
Duplication of basement membrane as in membranous glomerulonephritis, but without spikes.
These changes are due to ‘mesangial interposition’
Using electron microscopy and immunofluorescence, 2 types are identified.
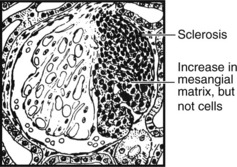
Focal Glomerulonephritis
In contrast to the glomerular diseases discussed already, focal glomerulonephritis affects only a proportion of glomeruli (focal) and only part of those glomeruli (segmental).
The pattern is associated with haematuria or nephrotic syndrome. Segmental lesions heal by fibrosis.
IgA Nephropathy
This is the commonest form of glomerulonephritis worldwide. It can present with microscopic or macroscopic haematuria or the nephrotic syndrome and may lead to chronic renal failure. It typically affects young males, who often suffer recurrent episodes after upper respiratory infections although all ages can be affected.
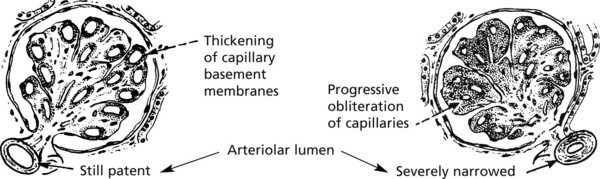
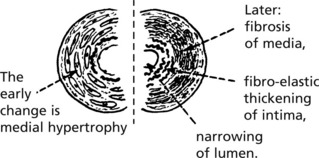
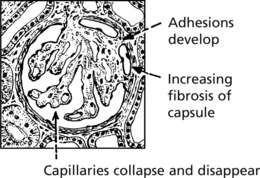
Chronic Glomerulonephritis (GN)
This is the end stage of many forms of glomerulonephritis, but most patients present at this stage without a history of previous renal disease.
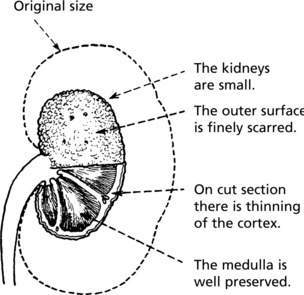
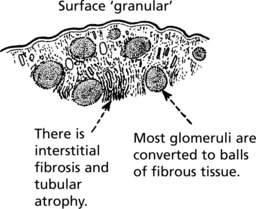
Pathology The kidneys are both small – granular contracted kidney.
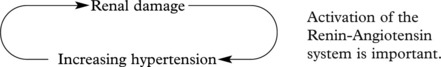
In chronic glomerulonephritis a vicious cycle is set up.
Glomerulonephritis – Disease Mechanisms
The mechanism in most forms of GN is:
Immune complexes can occur in glomeruli as follows:
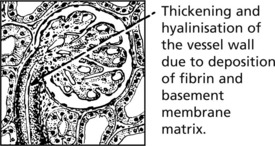
Glomerular Disease in Systemic Disorders
Systemic Lupus Erythematosus (SLE)
At least 50% of patients with SLE have clinical evidence of renal involvement, and almost all will have abnormalities on renal biopsy.
The renal changes form a spectrum.